Unique Growth Strategy in the Earth's First Trees Revealed in Silicified Fossil
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
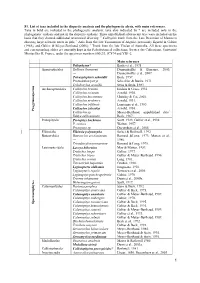
S1. List of Taxa Included in the Disparity Analysis and the Phylogenetic Alysis, with Main References
S1. List of taxa included in the disparity analysis and the phylogenetic alysis, with main references. Taxa in bold are included in the phylogenetic analysis; taxa also indicated by * are included only in the phylogenetic analysis and not in the disparity analysis. Three unpublished arborescent taxa were included on the basis that they showed additional anatomical diversity. 1 Callixylon trunk from the Late Devonian of Marrocco showing large sclerotic nests in pith; 2 Axis from the late Tournaisian of Algeria, previously figured in Galtier (1988), and Galtier & Meyer-Berthaud (2006); 3 Trunk from the late Viséan of Australia. All these specimens and corresponding slides are currently kept in the Paleobotanical collections, Service des Collections, Université Montpellier II, France, under the specimen numbers 600/2/3, JC874 and YB1-2. Main reference Psilophyton* Banks et al., 1975 Aneurophytales Rellimia thomsonii Dannenhoffer & Bonamo, 2003; --- Dannenhoffer et al., 2007. Tetraxylopteris schmidtii Beck, 1957. Proteokalon petryi Scheckler & Banks, 1971. Triloboxylon arnoldii Stein & Beck, 1983. s m Archaeopteridales Callixylon brownii Hoskin & Cross, 1951. r e Callixylon erianum Arnold, 1930. p s o Callixylon huronensis Chitaley & Cai, 2001. n Callixylon newberry Arnold, 1931. m y g Callixylon trifilievii Lemoigne et al., 1983. o r Callixylon zalesskyi Arnold, 1930. P Callixylon sp. Meyer-Berthaud, unpublished data1. Eddya sullivanensis Beck, 1967. Protopityales Protopitys buchiana Scott, 1923; Galtier et al., 1998. P. scotica Walton, 1957. Protopitys sp. Decombeix et al., 2005. Elkinsiales Elkinsia polymorpha Serbet & Rothwell, 1992. Buteoxylales Buteoxylon gordonianum Barnard &Long, 1973; Matten et al., --- 1980. Triradioxylon primaevum Barnard & Long, 1975. Lyginopteridales Laceya hibernica May & Matten, 1983. Tristichia longii Galtier, 1977. -

Earliest Record of Megaphylls and Leafy Structures, and Their Initial Diversification
Review Geology August 2013 Vol.58 No.23: 27842793 doi: 10.1007/s11434-013-5799-x Earliest record of megaphylls and leafy structures, and their initial diversification HAO ShouGang* & XUE JinZhuang Key Laboratory of Orogenic Belts and Crustal Evolution, School of Earth and Space Sciences, Peking University, Beijing 100871, China Received January 14, 2013; accepted February 26, 2013; published online April 10, 2013 Evolutionary changes in the structure of leaves have had far-reaching effects on the anatomy and physiology of vascular plants, resulting in morphological diversity and species expansion. People have long been interested in the question of the nature of the morphology of early leaves and how they were attained. At least five lineages of euphyllophytes can be recognized among the Early Devonian fossil plants (Pragian age, ca. 410 Ma ago) of South China. Their different leaf precursors or “branch-leaf com- plexes” are believed to foreshadow true megaphylls with different venation patterns and configurations, indicating that multiple origins of megaphylls had occurred by the Early Devonian, much earlier than has previously been recognized. In addition to megaphylls in euphyllophytes, the laminate leaf-like appendages (sporophylls or bracts) occurred independently in several dis- tantly related Early Devonian plant lineages, probably as a response to ecological factors such as high atmospheric CO2 concen- trations. This is a typical example of convergent evolution in early plants. Early Devonian, euphyllophyte, megaphyll, leaf-like appendage, branch-leaf complex Citation: Hao S G, Xue J Z. Earliest record of megaphylls and leafy structures, and their initial diversification. Chin Sci Bull, 2013, 58: 27842793, doi: 10.1007/s11434- 013-5799-x The origin and evolution of leaves in vascular plants was phology and evolutionary diversification of early leaves of one of the most important evolutionary events affecting the basal euphyllophytes remain enigmatic. -

Giant Cladoxylopsid Trees Resolve Enigma of the Earth's Earliest Forest Stumps at Gilboa
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/6385893 Giant cladoxylopsid trees resolve enigma of the Earth's earliest forest stumps at Gilboa Article in Nature · May 2007 DOI: 10.1038/nature05705 · Source: PubMed CITATIONS READS 91 254 5 authors, including: Frank Mannolini Linda VanAller Hernick New York State Museum New York State Museum 8 PUBLICATIONS 160 CITATIONS 9 PUBLICATIONS 253 CITATIONS SEE PROFILE SEE PROFILE Ed Landing Christopher Berry New York State Museum Cardiff University 244 PUBLICATIONS 3,365 CITATIONS 48 PUBLICATIONS 862 CITATIONS SEE PROFILE SEE PROFILE All content following this page was uploaded by Ed Landing on 06 February 2017. The user has requested enhancement of the downloaded file. All in-text references underlined in blue are added to the original document and are linked to publications on ResearchGate, letting you access and read them immediately. Vol 446 | 19 April 2007 | doi:10.1038/nature05705 LETTERS Giant cladoxylopsid trees resolve the enigma of the Earth’s earliest forest stumps at Gilboa William E. Stein1, Frank Mannolini2, Linda VanAller Hernick2, Ed Landing2 & Christopher M. Berry3 The evolution of trees of modern size growing together in forests Middle Devonian (Eifelian) into the Carboniferous, were major fundamentally changed terrestrial ecosystems1–3. The oldest trees contributors to floras worldwide14. Traditionally considered inter- are often thought to be of latest Devonian age (about 380–360 Myr mediate between Lower Devonian vascular plants and ferns or old) as indicated by the widespread occurrence of Archaeopteris sphenopsids, we do not yet understand these plants well enough to (Progymnospermopsida)4. -

Ancient Noeggerathialean Reveals the Seed Plant Sister Group Diversified Alongside the Primary Seed Plant Radiation
Ancient noeggerathialean reveals the seed plant sister group diversified alongside the primary seed plant radiation Jun Wanga,b,c,1, Jason Hiltond,e, Hermann W. Pfefferkornf, Shijun Wangg, Yi Zhangh, Jiri Beki, Josef Pšenickaˇ j, Leyla J. Seyfullahk, and David Dilcherl,m,1 aState Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing 210008, China; bCenter for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, Nanjing 210008, China; cUniversity of Chinese Academy of Sciences, Shijingshan District, Beijing 100049, China; dSchool of Geography, Earth and Environmental Sciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, United Kingdom; eBirmingham Institute of Forest Research, University of Birmingham, Edgbaston, Birmingham B15 2TT, United Kingdom; fDepartment of Earth and Environmental Science, University of Pennsylvania, Philadelphia, PA 19104-6316; gState Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Xiangshan, Beijing 100093, China; hCollege of Paleontology, Shenyang Normal University, Key Laboratory for Evolution of Past Life in Northeast Asia, Ministry of Natural Resources, Shenyang 110034, China; iDepartment of Palaeobiology and Palaeoecology, Institute of Geology v.v.i., Academy of Sciences of the Czech Republic, 165 00 Praha 6, Czech Republic; jCentre of Palaeobiodiversity, West Bohemian Museum in Plzen, 301 36 Plzen, Czech Republic; kDepartment of Paleontology, Geozentrum, University of Vienna, 1090 Vienna, Austria; lIndiana Geological and Water Survey, Bloomington, IN 47404; and mDepartment of Geology and Atmospheric Science, Indiana University, Bloomington, IN 47405 Contributed by David Dilcher, September 10, 2020 (sent for review July 2, 2020; reviewed by Melanie Devore and Gregory J. -

Archaeopteris Is the Earliest Known Modern Tree
letters to nature seawater nitrate mapping systems for use in open ocean and coastal waters. Deep-Sea Res. I 43, 1763± zones as important sites for the subsequent development of lateral 1775 (1996). 26. Obata, H., Karatani, H., Matsui, M. & Nakayama, E. Fundamental studies for chemical speciation in organs; and wood anatomy strategies that minimize the mechani- seawater with an improved analytical method. Mar. Chem. 56, 97±106 (1997). cal stresses caused by perennial branch growth. Acknowledgements. We thank G. Elrod, E. Guenther, C. Hunter, J. Nowicki and S. Tanner for the iron Archaeopteris is thought to have been an excurrent tree, with a analyses and assistance with sampling, and the crews of the research vessels Western Flyer and New Horizon single trunk producing helically arranged deciduous branches for providing valuable assistance at sea. Funding was provided by the David and Lucile Packard 7 Foundation through MBARI and by the National Science Foundation. growing almost horizontally . All studies relating to the develop- ment of Archaeopteris support the view that these ephemeral Correspondence and requests for materials should be addressed to K.S.J. (e-mail: johnson@mlml. calstate.edu). branches arise from the pseudomonopodial division of the trunk apex8±11. Apical branching also characterizes all other contempora- neous non-seed-plant taxa including those that had also evolved an arborescent habit, such as lepidosigillarioid lycopsids and cladoxy- Archaeopteris is the earliest lalean ferns. This pattern, which can disadvantage the tree if the trunk apex is damaged, contrasts with the axillary branching knownmoderntree reported in early seed plants12. Analysis of a 4 m-long trunk from the Famennian of Oklahoma has shown that Archaeopteris may Brigitte Meyer-Berthaud*, Stephen E. -

Classification, Molecular Phylogeny, Divergence Time, And
The JapaneseSocietyJapanese Society for Plant Systematics ISSN 1346-7565 Acta Phytotax. Geobot. 56 (2): 111-126 (2005) Invited article and Classification,MolecularPhylogeny,DivergenceTime, Morphological Evolution of Pteridophytes with Notes on Heterospory and and Monophyletic ParaphyleticGroups MASAHIRO KATO* Department ofBiotogicat Sciences,Graduate Schoot ofScience,Universitv. of7bkyo, Hongo, 7bk)]o IJ3- O033, lapan Pteridophytes are free-sporing vascular land plants that evolutionarily link bryophytes and seed plants. Conventiona], group (taxon)-based hierarchic classifications ofptcridophytes using phenetic characters are briefiy reviewcd. Review is also made for recent trcc-based cladistic analyses and molecular phy- logenetic analyses with increasingly large data sets ofmultiplc genes (compared to single genes in pre- vious studies) and increasingly large numbers of spccies representing major groups of pteridophytes (compared to particular groups in previous studies), and it is cxtended to most recent analyses of esti- mating divergcnce times ofpteridephytes, These c]assifications, phylogenetics, and divergcncc time esti- mates have improved our understanding of the diversity and historical structure of pteridophytes. Heterospory is noted with referencc to its origins, endospory, fertilization, and dispersal. Finally, menophylctic and paraphyletic groups rccently proposed or re-recognized are briefly dcscribcd. Key words: classification, divergence timc estimate. fems,heterospory, molecular phylogcny, pteri- dophytcs. Morphological -

Devonian As a Time of Major Innovation in Plants and Their Communities
1 Back to the Beginnings: The Silurian- 2 Devonian as a Time of Major Innovation 15 3 in Plants and Their Communities 4 Patricia G. Gensel, Ian Glasspool, Robert A. Gastaldo, 5 Milan Libertin, and Jiří Kvaček 6 Abstract Silurian, with the Early Silurian Cooksonia barrandei 31 7 Massive changes in terrestrial paleoecology occurred dur- from central Europe representing the earliest vascular 32 8 ing the Devonian. This period saw the evolution of both plant known, to date. This plant had minute bifurcating 33 9 seed plants (e.g., Elkinsia and Moresnetia), fully lami- aerial axes terminating in expanded sporangia. Dispersed 34 10 nate∗ leaves and wood. Wood evolved independently in microfossils (spores and phytodebris) in continental and 35AU2 11 different plant groups during the Middle Devonian (arbo- coastal marine sediments provide the earliest evidence for 36 12 rescent lycopsids, cladoxylopsids, and progymnosperms) land plants, which are first reported from the Early 37 13 resulting in the evolution of the tree habit at this time Ordovician. 38 14 (Givetian, Gilboa forest, USA) and of various growth and 15 architectural configurations. By the end of the Devonian, 16 30-m-tall trees were distributed worldwide. Prior to the 17 appearance of a tree canopy habit, other early plant groups 15.1 Introduction 39 18 (trimerophytes) that colonized the planet’s landscapes 19 were of smaller stature attaining heights of a few meters Patricia G. Gensel and Milan Libertin 40 20 with a dense, three-dimensional array of thin lateral 21 branches functioning as “leaves”. Laminate leaves, as we We are now approaching the end of our journey to vegetated 41 AU3 22 now know them today, appeared, independently, at differ- landscapes that certainly are unfamiliar even to paleontolo- 42 23 ent times in the Devonian. -

Diversity and Evolution of the Megaphyll in Euphyllophytes
G Model PALEVO-665; No. of Pages 16 ARTICLE IN PRESS C. R. Palevol xxx (2012) xxx–xxx Contents lists available at SciVerse ScienceDirect Comptes Rendus Palevol w ww.sciencedirect.com General palaeontology, systematics and evolution (Palaeobotany) Diversity and evolution of the megaphyll in Euphyllophytes: Phylogenetic hypotheses and the problem of foliar organ definition Diversité et évolution de la mégaphylle chez les Euphyllophytes : hypothèses phylogénétiques et le problème de la définition de l’organe foliaire ∗ Adèle Corvez , Véronique Barriel , Jean-Yves Dubuisson UMR 7207 CNRS-MNHN-UPMC, centre de recherches en paléobiodiversité et paléoenvironnements, 57, rue Cuvier, CP 48, 75005 Paris, France a r t i c l e i n f o a b s t r a c t Article history: Recent paleobotanical studies suggest that megaphylls evolved several times in land plant st Received 1 February 2012 evolution, implying that behind the single word “megaphyll” are hidden very differ- Accepted after revision 23 May 2012 ent notions and concepts. We therefore review current knowledge about diverse foliar Available online xxx organs and related characters observed in fossil and living plants, using one phylogenetic hypothesis to infer their origins and evolution. Four foliar organs and one lateral axis are Presented by Philippe Taquet described in detail and differ by the different combination of four main characters: lateral organ symmetry, abdaxity, planation and webbing. Phylogenetic analyses show that the Keywords: “true” megaphyll appeared at least twice in Euphyllophytes, and that the history of the Euphyllophytes Megaphyll four main characters is different in each case. The current definition of the megaphyll is questioned; we propose a clear and accurate terminology in order to remove ambiguities Bilateral symmetry Abdaxity of the current vocabulary. -

Synoptic Taxonomy of Major Fossil Groups
APPENDIX Synoptic Taxonomy of Major Fossil Groups Important fossil taxa are listed down to the lowest practical taxonomic level; in most cases, this will be the ordinal or subordinallevel. Abbreviated stratigraphic units in parentheses (e.g., UCamb-Ree) indicate maximum range known for the group; units followed by question marks are isolated occurrences followed generally by an interval with no known representatives. Taxa with ranges to "Ree" are extant. Data are extracted principally from Harland et al. (1967), Moore et al. (1956 et seq.), Sepkoski (1982), Romer (1966), Colbert (1980), Moy-Thomas and Miles (1971), Taylor (1981), and Brasier (1980). KINGDOM MONERA Class Ciliata (cont.) Order Spirotrichia (Tintinnida) (UOrd-Rec) DIVISION CYANOPHYTA ?Class [mertae sedis Order Chitinozoa (Proterozoic?, LOrd-UDev) Class Cyanophyceae Class Actinopoda Order Chroococcales (Archean-Rec) Subclass Radiolaria Order Nostocales (Archean-Ree) Order Polycystina Order Spongiostromales (Archean-Ree) Suborder Spumellaria (MCamb-Rec) Order Stigonematales (LDev-Rec) Suborder Nasselaria (Dev-Ree) Three minor orders KINGDOM ANIMALIA KINGDOM PROTISTA PHYLUM PORIFERA PHYLUM PROTOZOA Class Hexactinellida Order Amphidiscophora (Miss-Ree) Class Rhizopodea Order Hexactinosida (MTrias-Rec) Order Foraminiferida* Order Lyssacinosida (LCamb-Rec) Suborder Allogromiina (UCamb-Ree) Order Lychniscosida (UTrias-Rec) Suborder Textulariina (LCamb-Ree) Class Demospongia Suborder Fusulinina (Ord-Perm) Order Monaxonida (MCamb-Ree) Suborder Miliolina (Sil-Ree) Order Lithistida -

Megaphylls, Microphylls and the Evolution of Leaf Development
Opinion Megaphylls, microphylls and the evolution of leaf development Alexandru M.F. Tomescu Department of Biological Sciences, Humboldt State University, Arcata, CA 95521, USA Originally coined to emphasize morphological differ- However, the microphyll–megaphyll divide is not as ences, ‘microphyll’ and ‘megaphyll’ became synon- clear cut, and morphological definitions that contrast ymous with the idea that vascular plant leaves are not microphylls and megaphylls as mutually exclusive con- homologous. Although it is now accepted that leaves cepts of leaves are inconsistent. Moreover, current under- evolved independently in several euphyllophyte standing of plant phylogeny and leaf development fails to lineages, ‘megaphyll’ has grown to reflect another type shed light on the origin of microphylls, and supports of homology, that of euphyllophyte leaf precursor struc- several independent origins of megaphylls. Here, I review tures. However, evidence from the fossil record and plant phylogeny and developmental data from fossil and developmental pathways fails to indicate homology extant trachephytes, as well as the current understanding and suggests homoplasy of precursor structures. Thus, of genetic pathways controlling leaf development, to argue as I discuss here, ‘megaphyll’ should be abandoned that the megaphyll concept should be abandoned because because it perpetuates an unsupported idea of it perpetuates misconceptions and confusion based on homology, leading to misconceptions that pervade plant unsupported homology. biology thinking and can bias hypothesis and inference in developmental and phylogenetic studies. Alternative Morphological inconsistencies and overlap in the definitions are needed that are based on development microphyll–megaphyll dichotomy and phylogeny for different independently evolved leaf Microphylls are defined as leaves of small size, with simple types. -

The Terrestrialization Process: a Palaeobotanical and Palynological Perspective Brigitte Meyer-Berthaud, Thomas Servais, Marco Vecoli, Philippe Gerrienne
The terrestrialization process: A palaeobotanical and palynological perspective Brigitte Meyer-Berthaud, Thomas Servais, Marco Vecoli, Philippe Gerrienne To cite this version: Brigitte Meyer-Berthaud, Thomas Servais, Marco Vecoli, Philippe Gerrienne. The terrestrialization process: A palaeobotanical and palynological perspective. Review of Palaeobotany and Palynology, Elsevier, 2016, The terrestrialization process : a palaeobotanical and palynological perspective - volume 1, 224 (1), pp.1-3. 10.1016/j.revpalbo.2015.10.011. hal-01278369 HAL Id: hal-01278369 https://hal-sde.archives-ouvertes.fr/hal-01278369 Submitted on 26 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Review of Palaeobotany and Palynology 224 (2016) 1–3 Contents lists available at ScienceDirect Review of Palaeobotany and Palynology journal homepage: www.elsevier.com/locate/revpalbo The terrestrialization process: A palaeobotanical and palynological perspective Brigitte Meyer-Berthaud a,⁎,ThomasServaisb,MarcoVecolic,PhilippeGerrienned a CNRS, Université de Montpellier, Botanique -

Stepwise Evolution of Paleozoic Tracheophytes from South China: Contrasting Leaf Disparity and Taxic Diversity
Earth-Science Reviews 148 (2015) 77–93 Contents lists available at ScienceDirect Earth-Science Reviews journal homepage: www.elsevier.com/locate/earscirev Stepwise evolution of Paleozoic tracheophytes from South China: Contrasting leaf disparity and taxic diversity Jinzhuang Xue a,⁎,PuHuanga,MarcelloRutab, Michael J. Benton c, Shougang Hao a,⁎, Conghui Xiong d, Deming Wang a, Borja Cascales-Miñana e, Qi Wang f,LeLiua a The Key Laboratory of Orogenic Belts and Crustal Evolution, School of Earth and Space Sciences, Peking University, Beijing 100871, PR China b School of Life Sciences, University of Lincoln, Lincoln LN6 7DL, UK c School of Earth Sciences, University of Bristol, Bristol BS8 1RJ, UK d School of Earth Sciences, Lanzhou University, Lanzhou 730000, PR China e PPP, Département de Géologie, Université de Liège, B-4000 Liège, Belgium f State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, PR China article info abstract Article history: During the late Paleozoic, vascular land plants (tracheophytes) diversified into a remarkable variety of morpho- Received 15 February 2015 logical types, ranging from tiny, aphyllous, herbaceous forms to giant leafy trees. Leaf shape is a key determinant Accepted 26 May 2015 of both function and structural diversity of plants, but relatively little is known about the tempo and mode of leaf Available online 30 May 2015 morphological diversification and its correlation with tracheophyte diversity and abiotic changes during this re- markable macroevolutionary event, the greening of the continents. We use the extensive record of Paleozoic tra- Keywords: fi Leaf morphology cheophytes from South China to explore models of morphological evolution in early land plants.