Megaphylls, Microphylls and the Evolution of Leaf Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gene Expression Data Support the Hypothesis That Isoetes Rootlets Are True Roots and Not Modifed Leaves Alexander J
www.nature.com/scientificreports OPEN Gene expression data support the hypothesis that Isoetes rootlets are true roots and not modifed leaves Alexander J. Hetherington1,2, David M. Emms1, Steven Kelly1 & Liam Dolan1,3* Rhizomorphic lycopsids are the land plant group that includes the frst giant trees to grow on Earth and extant species in the genus Isoetes. Two mutually exclusive hypotheses account for the evolution of terminal rooting axes called rootlets among the rhizomorphic lycopsids. One hypothesis states that rootlets are true roots, like roots in other lycopsids. The other states that rootlets are modifed leaves. Here we test predictions of each hypothesis by investigating gene expression in the leaves and rootlets of Isoetes echinospora. We assembled the de novo transcriptome of axenically cultured I. echinospora. Gene expression signatures of I. echinospora rootlets and leaves were diferent. Furthermore, gene expression signatures of I. echinospora rootlets were similar to gene expression signatures of true roots of Selaginella moellendorfi and Arabidopsis thaliana. RSL genes which positively regulate cell diferentiation in roots were either exclusively or preferentially expressed in the I. echinospora rootlets, S. moellendorfi roots and A. thaliana roots compared to the leaves of each respective species. Taken together, gene expression data from the de-novo transcriptome of I. echinospora are consistent with the hypothesis that Isoetes rootlets are true roots and not modifed leaves. Te frst giant (> 50 m) trees to grow on Earth, the arborescent clubmosses, were tethered to the ground by rooting structures termed stigmarian systems whose homology has been debated for more than 150 years1–9. Stigmarian rooting systems consisted of two components, a central axis (rhizomorph) on which developed large numbers of fne axes (rootlets). -

American Fern Journal
AMERICAN FERN JOURNAL QUARTERLY JOURNAL OF THE AMERICAN FERN SOCIETY Isoetes duriei New to Lebanon LYTTON J. MUSSELMAN and MOHAMMAD S. AL- ZEIN, Department of Biological Sciences, Old Dominion University, Norfolk, Virginia 23529-0266, USA. American Fern Journal 99(4):333–334 (2009) SHORTER NOTES Isoetes duriei New to Lebanon.—In a recent paper, we (Bolin et al., Turkish Journal of Botany 32:447–457. 2008) discussed the taxonomy and distribution of the quillworts (species of the genus Isoetes, Lycophyta) in Western Asia. In this supplementary note, we record the presence of three quillworts new to Lebanon –one a widespread Mediterranean species, one known from only a single site in Turkey and two in Syria, and an undescribed new species. With this report, the number of documented species in Lebanon has increased from one to three. Voucher specimens will be deposited at BEI, E, and ODU. In his flora, Mouterde (Nouvelle Flora du Liban et de la Syria. Beirut: Editions de L’Imprimerie Catholique. 1966) included two species of Isoetes from Syria and Lebanon– Isoetes olympica A. Braun known from only a few sites on Jebel Al Arab (historically known as Jebel Druze) in extreme southeastern Syria, and what Mouterde called I. histrix Bory forma subinermis Durieu from the Akkar region of northern Lebanon. He separated the two species chiefly on the basis of velum coverage—I. olympica with an incomplete velum and I. histrix forma subinermis has complete velum coverage. Musselman (Fern Gaz. 16(6, 7 & 8):324–3 29. 2002) noted the impending demise of the Jebel Al Arab populations due to habitat destruction. -

WRA Species Report
Family: Selaginellaceae Taxon: Selaginella braunii Synonym: Lycopodioides braunii (Baker) Kuntze Common Name: arborvitae fern Selaginella braunii fo. hieronymi Alderw. Braun's spike-moss Selaginella hieronymi Alderw. Chinese lace-fern spike-moss Selaginella vogelii Mett. treelet spike-moss Questionaire : current 20090513 Assessor: Assessor Designation: H(HPWRA) Status: Assessor Approved Data Entry Person: Assessor WRA Score 7 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- Low substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 y 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 n 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 y 301 Naturalized beyond native range y = 1*multiplier (see y Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see n Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see n Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see n Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see y Appendix 2) 401 Produces spines, thorns or -

Tiny Fossil Sheds Light on Miniaturization of Birds
Retraction Tiny fossil sheds light on miniaturization of birds Roger B. J. Benson Nature 579, 199–200 (2020) In view of the fact that the authors of ‘Hummingbird-sized dinosaur from the Cretaceous period of Myanmar‘ (L. Xing et al. Nature 579, 245–249; 2020) are retracting their report, I wish to retract this News & Views article, which dealt with this study and was based on the accuracy and reproducibility of their data. Nature | 22 July 2020 ©2020 Spri nger Nature Li mited. All rights reserved. drives the assembly of DNA-PK and stimulates regulation of protein synthesis. And, although 3. Dragon, F. et al. Nature 417, 967–970 (2002). its catalytic activity in vitro, although does so further studies are required, we might have 4. Adelmant, G. et al. Mol. Cell. Proteom. 11, 411–421 (2012). much less efficiently than can DNA. taken a step closer to deciphering the 5. Britton, S., Coates, J. & Jackson, S. P. J. Cell Biol. 202, Taken together, these observations suggest mysterious ribosomopathies. 579–595 (2013). a model in which KU recruits DNA-PKcs to the 6. Barandun, J. et al. Nature Struct. Mol. Biol. 24, 944–953 (2017). small-subunit processome. In the case of Alan J. Warren is at the Cambridge Institute 7. Ma, Y. et al. Cell 108, 781–794 (2002). kinase-defective DNA-PK, the mutant enzyme’s for Medical Research, Hills Road, Cambridge 8. Yin, X. et al. Cell Res. 27, 1341–1350 (2017). inability to regulate its own activity gives the CB2 OXY, UK. 9. Sharif, H. et al. -

The Origin and Early Evolution of Vascular Plant Shoots and Leaves Rstb.Royalsocietypublishing.Org C
Downloaded from http://rstb.royalsocietypublishing.org/ on January 22, 2018 The origin and early evolution of vascular plant shoots and leaves rstb.royalsocietypublishing.org C. Jill Harrison 1 and Jennifer L. Morris 2 1School of Biological Sciences, and 2School of Earth Sciences, University of Bristol, 24 Tyndall Avenue, Bristol BS8 1TQ, UK Review CJH, 0000-0002-5228-600X; JLM, 0000-0002-7453-3841 Cite this article: Harrison CJ, Morris JL. 2017 The morphology of plant fossils from the Rhynie chert has generated long- standing questions about vascular plant shoot and leaf evolution, for The origin and early evolution of vascular plant instance, which morphologies were ancestral within land plants, when did shoots and leaves. Phil. Trans. R. Soc. B 373 : vascular plants first arise and did leaves have multiple evolutionary origins? 20160496. Recent advances combining insights from molecular phylogeny, palaeobotany http://dx.doi.org/10.1098/rstb.2016.0496 and evo–devo research address these questions and suggest the sequence of morphological innovation during vascular plant shoot and leaf evolution. The evidence pinpoints testable developmental and genetic hypotheses relat- Accepted: 11 August 2017 ing to the origin of branching and indeterminate shoot architectures prior to the evolution of leaves, and demonstrates underestimation of polyphyly in One contribution of 18 to a discussion meeting the evolution of leaves from branching forms in ‘telome theory’ hypotheses issue ‘The Rhynie cherts: our earliest terrestrial of leaf evolution. This review discusses fossil, developmental and genetic ecosystem revisited’. evidence relating to the evolution of vascular plant shoots and leaves in a phylogenetic framework. This article is part of a discussion meeting issue ‘The Rhynie cherts: our Subject Areas: earliest terrestrial ecosystem revisited’. -

Selaginellaceae: Traditional Use, Phytochemistry and Pharmacology
MS Editions BOLETIN LATINOAMERICANO Y DEL CARIBE DE PLANTAS MEDICINALES Y AROMÁTICAS 19 (3): 247 - 288 (2020) © / ISSN 0717 7917 / www.blacpma.ms-editions.cl Revisión | Review Selaginellaceae: traditional use, phytochemistry and pharmacology [Selaginellaceae: uso tradicional, fitoquímica y farmacología] Fernanda Priscila Santos Reginaldo, Isabelly Cristina de Matos Costa & Raquel Brandt Giordani College of Pharmacy, Pharmacy Department. University of Rio Grande do Norte, Natal, RN, Brazil. Contactos | Contacts: Raquel Brandt GIORDANI - E-mail address: [email protected] Abstract: Selaginella is the only genus from Selaginellaceae, and it is considered a key factor in studying evolution. The family managed to survive the many biotic and abiotic pressures during the last 400 million years. The purpose of this review is to provide an up-to-date overview of Selaginella in order to recognize their potential and evaluate future research opportunities. Carbohydrates, pigments, steroids, phenolic derivatives, mainly flavonoids, and alkaloids are the main natural products in Selaginella. A wide spectrum of in vitro and in vivo pharmacological activities, some of them pointed out by folk medicine, has been reported. Future studies should afford valuable new data on better explore the biological potential of the flavonoid amentoflavone and their derivatives as chemical bioactive entities; develop studies about toxicity and, finally, concentrate efforts on elucidate mechanisms of action for biological properties already reported. Keywords: Selaginella; Natural Products; Overview. Resumen: Selaginella es el único género de Selaginellaceae, y se considera un factor clave en el estudio de la evolución. La familia logró sobrevivir a las muchas presiones bióticas y abióticas durante los últimos 400 millones de años. -

Getting to the Roots: a Developmental Genetic View of Root Anatomy and Function from Arabidopsis to Lycophytes
fpls-09-01410 September 21, 2018 Time: 17:3 # 1 REVIEW published: 25 September 2018 doi: 10.3389/fpls.2018.01410 Getting to the Roots: A Developmental Genetic View of Root Anatomy and Function From Arabidopsis to Lycophytes Frauke Augstein and Annelie Carlsbecker* Department of Organismal Biology, Physiological Botany and Linnean Centre for Plant Biology in Uppsala, Uppsala University, Uppsala, Sweden Roots attach plants to the ground and ensure efficient and selective uptake of water and nutrients. These functions are facilitated by the morphological and anatomical structures of the root, formed by the activity of the root apical meristem (RAM) and consecutive patterning and differentiation of specific tissues with distinct functions. Despite the importance of this plant organ, its evolutionary history is not clear, but fossils suggest that roots evolved at least twice, in the lycophyte (clubmosses and their allies) and in the euphyllophyte (ferns and seed plants) lineages. Both lycophyte and euphyllophyte roots grow indeterminately by the action of an apical meristem, which is protected by a root cap. They produce root hairs, and in most species the vascular stele is Edited by: guarded by a specialized endodermal cell layer. Hence, most of these traits must have Annette Becker, evolved independently in these lineages. This raises the question if the development Justus Liebig Universität Gießen, Germany of these apparently analogous tissues is regulated by distinct or homologous genes, Reviewed by: independently recruited from a common ancestor of lycophytes and euphyllophytes. Hongchang Cui, Currently, there are few studies of the genetic and molecular regulation of lycophyte Florida State University, United States and fern roots. -
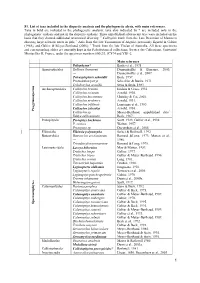
S1. List of Taxa Included in the Disparity Analysis and the Phylogenetic Alysis, with Main References
S1. List of taxa included in the disparity analysis and the phylogenetic alysis, with main references. Taxa in bold are included in the phylogenetic analysis; taxa also indicated by * are included only in the phylogenetic analysis and not in the disparity analysis. Three unpublished arborescent taxa were included on the basis that they showed additional anatomical diversity. 1 Callixylon trunk from the Late Devonian of Marrocco showing large sclerotic nests in pith; 2 Axis from the late Tournaisian of Algeria, previously figured in Galtier (1988), and Galtier & Meyer-Berthaud (2006); 3 Trunk from the late Viséan of Australia. All these specimens and corresponding slides are currently kept in the Paleobotanical collections, Service des Collections, Université Montpellier II, France, under the specimen numbers 600/2/3, JC874 and YB1-2. Main reference Psilophyton* Banks et al., 1975 Aneurophytales Rellimia thomsonii Dannenhoffer & Bonamo, 2003; --- Dannenhoffer et al., 2007. Tetraxylopteris schmidtii Beck, 1957. Proteokalon petryi Scheckler & Banks, 1971. Triloboxylon arnoldii Stein & Beck, 1983. s m Archaeopteridales Callixylon brownii Hoskin & Cross, 1951. r e Callixylon erianum Arnold, 1930. p s o Callixylon huronensis Chitaley & Cai, 2001. n Callixylon newberry Arnold, 1931. m y g Callixylon trifilievii Lemoigne et al., 1983. o r Callixylon zalesskyi Arnold, 1930. P Callixylon sp. Meyer-Berthaud, unpublished data1. Eddya sullivanensis Beck, 1967. Protopityales Protopitys buchiana Scott, 1923; Galtier et al., 1998. P. scotica Walton, 1957. Protopitys sp. Decombeix et al., 2005. Elkinsiales Elkinsia polymorpha Serbet & Rothwell, 1992. Buteoxylales Buteoxylon gordonianum Barnard &Long, 1973; Matten et al., --- 1980. Triradioxylon primaevum Barnard & Long, 1975. Lyginopteridales Laceya hibernica May & Matten, 1983. Tristichia longii Galtier, 1977. -

<I>Equisetum Giganteum</I>
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 3-24-2009 Ecophysiology and Biomechanics of Equisetum Giganteum in South America Chad Eric Husby Florida International University, [email protected] DOI: 10.25148/etd.FI10022522 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Recommended Citation Husby, Chad Eric, "Ecophysiology and Biomechanics of Equisetum Giganteum in South America" (2009). FIU Electronic Theses and Dissertations. 200. https://digitalcommons.fiu.edu/etd/200 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida ECOPHYSIOLOGY AND BIOMECHANICS OF EQUISETUM GIGANTEUM IN SOUTH AMERICA A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in BIOLOGY by Chad Eric Husby 2009 To: Dean Kenneth Furton choose the name of dean of your college/school College of Arts and Sciences choose the name of your college/school This dissertation, written by Chad Eric Husby, and entitled Ecophysiology and Biomechanics of Equisetum Giganteum in South America, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. _______________________________________ Bradley C. Bennett _______________________________________ Jack B. Fisher _______________________________________ David W. Lee _______________________________________ Leonel Da Silveira Lobo O'Reilly Sternberg _______________________________________ Steven F. Oberbauer, Major Professor Date of Defense: March 24, 2009 The dissertation of Chad Eric Husby is approved. -

Tansley Review Evolution of Development of Vascular Cambia and Secondary Growth
New Phytologist Review Tansley review Evolution of development of vascular cambia and secondary growth Author for correspondence: Rachel Spicer1 and Andrew Groover2 Andrew Groover 1The Rowland Institute at Harvard, Cambridge, MA, USA; 2Institute of Forest Genetics, Pacific Tel: +1 530 759 1738 Email: [email protected] Southwest Research Station, USDA Forest Service, Davis, CA, USA Received: 29 December 2009 Accepted: 14 February 2010 Contents Summary 577 V. Evolution of development approaches for the study 587 of secondary vascular growth I. Introduction 577 VI. Conclusions 589 II. Generalized function of vascular cambia and their 578 developmental and evolutionary origins Acknowledgements 589 III. Variation in secondary vascular growth in angiosperms 581 References 589 IV. Genes and mechanisms regulating secondary vascular 584 growth and their evolutionary origins Summary New Phytologist (2010) 186: 577–592 Secondary growth from vascular cambia results in radial, woody growth of stems. doi: 10.1111/j.1469-8137.2010.03236.x The innovation of secondary vascular development during plant evolution allowed the production of novel plant forms ranging from massive forest trees to flexible, Key words: forest trees, genomics, Populus, woody lianas. We present examples of the extensive phylogenetic variation in sec- wood anatomy, wood formation. ondary vascular growth and discuss current knowledge of genes that regulate the development of vascular cambia and woody tissues. From these foundations, we propose strategies for genomics-based research in the evolution of development, which is a next logical step in the study of secondary growth. I. Introduction this pattern characterizes most extant forest trees, significant variation exists among taxa, ranging from extinct woody Secondary vascular growth provides a means of radially lycopods and horsetails with unifacial cambia (Cichan & thickening and strengthening plant axes initiated during Taylor, 1990; Willis & McElwain, 2002), to angiosperms primary, or apical growth. -

The Big Bloom—How Flowering Plants Changed the World
The Big Bloom—How Flowering Plants Changed the World Written by Michael Klesius Republished from the pages of National Geographic magazine -- July 2002 In the summer of 1973 sunflowers appeared in my father's vegetable garden. They seemed to sprout overnight in a few rows he had lent that year to new neighbors from California. Only six years old at the time, I was at first put off by these garish plants. Such strange and vibrant flowers seemed out of place among the respectable beans, peppers, spinach, and other vegetables we had always grown. Gradually, however, the brilliance of the sunflowers won me over. Their fiery halos relieved the green monotone that by late summer ruled the garden. I marveled at birds that clung upside down to the shaggy, gold disks, wings fluttering, looting the seeds. Sunflowers defined flowers for me that summer and changed my view of the world. Flowers have a way of doing that. They began changing the way the world looked almost as soon as they appeared on Earth about 130 million years ago, during the Cretaceous period. That's relatively recent in geologic time: If all Earth's history were compressed into an hour, flowering plants would exist for only the last 90 seconds. But once they took firm root about 100 million years ago, they swiftly diversified in an explosion of varieties that established most of the flowering plant families of the modern world. Today flowering plant species outnumber by twenty to one those of ferns and cone-bearing trees, or conifers, which had thrived for 200 million years before the first bloom appeared. -

Earliest Record of Megaphylls and Leafy Structures, and Their Initial Diversification
Review Geology August 2013 Vol.58 No.23: 27842793 doi: 10.1007/s11434-013-5799-x Earliest record of megaphylls and leafy structures, and their initial diversification HAO ShouGang* & XUE JinZhuang Key Laboratory of Orogenic Belts and Crustal Evolution, School of Earth and Space Sciences, Peking University, Beijing 100871, China Received January 14, 2013; accepted February 26, 2013; published online April 10, 2013 Evolutionary changes in the structure of leaves have had far-reaching effects on the anatomy and physiology of vascular plants, resulting in morphological diversity and species expansion. People have long been interested in the question of the nature of the morphology of early leaves and how they were attained. At least five lineages of euphyllophytes can be recognized among the Early Devonian fossil plants (Pragian age, ca. 410 Ma ago) of South China. Their different leaf precursors or “branch-leaf com- plexes” are believed to foreshadow true megaphylls with different venation patterns and configurations, indicating that multiple origins of megaphylls had occurred by the Early Devonian, much earlier than has previously been recognized. In addition to megaphylls in euphyllophytes, the laminate leaf-like appendages (sporophylls or bracts) occurred independently in several dis- tantly related Early Devonian plant lineages, probably as a response to ecological factors such as high atmospheric CO2 concen- trations. This is a typical example of convergent evolution in early plants. Early Devonian, euphyllophyte, megaphyll, leaf-like appendage, branch-leaf complex Citation: Hao S G, Xue J Z. Earliest record of megaphylls and leafy structures, and their initial diversification. Chin Sci Bull, 2013, 58: 27842793, doi: 10.1007/s11434- 013-5799-x The origin and evolution of leaves in vascular plants was phology and evolutionary diversification of early leaves of one of the most important evolutionary events affecting the basal euphyllophytes remain enigmatic.