Selaginellaceae: Traditional Use, Phytochemistry and Pharmacology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WRA Species Report
Family: Selaginellaceae Taxon: Selaginella braunii Synonym: Lycopodioides braunii (Baker) Kuntze Common Name: arborvitae fern Selaginella braunii fo. hieronymi Alderw. Braun's spike-moss Selaginella hieronymi Alderw. Chinese lace-fern spike-moss Selaginella vogelii Mett. treelet spike-moss Questionaire : current 20090513 Assessor: Assessor Designation: H(HPWRA) Status: Assessor Approved Data Entry Person: Assessor WRA Score 7 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- Low substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 y 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 n 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 y 301 Naturalized beyond native range y = 1*multiplier (see y Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see n Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see n Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see n Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see y Appendix 2) 401 Produces spines, thorns or -

Evidence-Based Medicinal Potential and Possible Role of Selaginella in the Prevention of Modern Chronic Diseases: Ethnopharmacological and Ethnobotanical Perspective
REVIEW ARTICLE Rec. Nat. Prod. X:X (2021) XX-XX Evidence-Based Medicinal Potential and Possible Role of Selaginella in the Prevention of Modern Chronic Diseases: Ethnopharmacological and Ethnobotanical Perspective Mohd Adnan 1*, Arif Jamal Siddiqui 1, Arshad Jamal 1, Walid Sabri Hamadou 1, Amir Mahgoub Awadelkareem 2, Manojkumar Sachidanandan 3 and Mitesh Patel 4 1Department of Biology, College of Science, University of Ha’il, Ha’il, P O Box 2440, Saudi Arabia 2Department of Clinical Nutrition, College of Applied Medial Sciences, University of Hail, Hail PO Box 2440, Saudi Arabia 3Department of Oral Radiology, College of Dentistry, University of Hail, Hail, PO Box 2440, Saudi Arabia 4Bapalal Vaidya Botanical Research Centre, Department of Biosciences, Veer Narmad South Gujarat University, Surat, Gujarat, India (Received November 26, 2020; Revised January 29, 2021; Accepted January 31, 2021) Abstract: Different species of the genus Selaginella are exploited for various ethnomedicinal purposes around the globe; mainly to cure fever, jaundice, hepatic disorders, cardiac diseases, cirrhosis, diarrhea, cholecystitis, sore throat, cough of lungs, promotes blood circulation, removes blood stasis and stops external bleeding after trauma and separation of the umbilical cord. Though, high content of various phytochemicals has been isolated from Selaginella species, flavonoids have been recognized as the most active component in the genus. Crude extract and different bioactive compounds of this plant have revealed various in vitro bioactivities such as, antimicrobial, antiviral, anti-diabetic, anti-mutagenic, anti-inflammatory, anti-nociceptive, anti-spasmodic, anticancer and anti-Alzheimer. However, more studies into the pharmacological activities are needed, since none of the professed bioactivity of this plant have ever been fully evaluated. -

Chapter 23: the Early Tracheophytes
Chapter 23 The Early Tracheophytes THE LYCOPHYTES Lycopodium Has a Homosporous Life Cycle Selaginella Has a Heterosporous Life Cycle Heterospory Allows for Greater Parental Investment Isoetes May Be the Only Living Member of the Lepidodendrid Group THE MONILOPHYTES Whisk Ferns Ophioglossalean Ferns Horsetails Marattialean Ferns True Ferns True Fern Sporophytes Typically Have Underground Stems Sexual Reproduction Usually Is Homosporous Fern Have a Variety of Alternative Means of Reproduction Ferns Have Ecological and Economic Importance SUMMARY PLANTS, PEOPLE, AND THE ENVIRONMENT: Sporophyte Prominence and Survival on Land PLANTS, PEOPLE, AND THE ENVIRONMENT: Coal, Smog, and Forest Decline THE OCCUPATION OF THE LAND PLANTS, PEOPLE, AND THE The First Tracheophytes Were ENVIRONMENT: Diversity Among the Ferns Rhyniophytes Tracheophytes Became Increasingly Better PLANTS, PEOPLE, AND THE Adapted to the Terrestrial Environment ENVIRONMENT: Fern Spores Relationships among Early Tracheophytes 1 KEY CONCEPTS 1. Tracheophytes, also called vascular plants, possess lignified water-conducting tissue (xylem). Approximately 14,000 species of tracheophytes reproduce by releasing spores and do not make seeds. These are sometimes called seedless vascular plants. Tracheophytes differ from bryophytes in possessing branched sporophytes that are dominant in the life cycle. These sporophytes are more tolerant of life on dry land than those of bryophytes because water movement is controlled by strongly lignified vascular tissue, stomata, and an extensive cuticle. The gametophytes, however still require a seasonally wet habitat, and water outside the plant is essential for the movement of sperm from antheridia to archegonia. 2. The rhyniophytes were the first tracheophytes. They consisted of dichotomously branching axes, lacking roots and leaves. They are all extinct. -
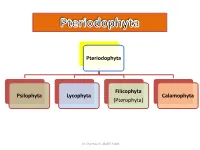
Pteriodophyta Psilophyta Lycophyta Filicophyta (Pterophyta) Calamophyta
Pteriodophyta Filicophyta Psilophyta Lycophyta Calamophyta (Pterophyta) Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora (Eligulatae) Order: Lycopodiales Family: Lycopodiaceae e.g. Lycopodium Subclass(2): Heterospora (Ligulatae) Order: Selaginellales Family: Selaginellaceae e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Phylum: Lepidophyta • It is characterized by: 1. The plant is differentiated into stem, leaves and roots. 2. Leaves are microphyllous (small, one vein and with no leaf gap). 3. The stele is protostele, siphonostele or polystele. 4. Protoxylem is exarch (ranging from a complete external cylinder to a polyarch stele in which there are many protoxylem ribs). 5. Sporangia are solitary, carried on special leaves (sporophylls). Sporophylls are usually collected in strobili. Dr. Shaimaa N. Abd El-Fatah . Among this phylum there are two independent evolutionary lines Heterospora (Ligulatae) Homospora (Eligulatae) • Characterized by presence • Eligulate (ligule lacking). of ligule (small outgrowth on • Homosporous (one type of the upper surface of the spores). leaf). • Heterosporous character (2 types of spores: microspores-- small, give rise to male gametophyte. & megaspores– larger, give rise to female gametophyte.) Dr. Shaimaa N. Abd El-Fatah • The phylum includes one class: Lycopodinae. • The class is classified into 4 orders: • Plants belong to the first order are homosporae (eligulatae), while the remaining three are heterosporae (ligulatae). Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora Subclass(2): Heterospora (Eligulatae) (Ligulatae) Order: Lycopodiales Order: Selaginellales Family: Lycopodiaceae Family: Selaginellaceae e.g. Lycopodium e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Lycopodiales This order is characterized by: 1. Homosporous and eligulatae. 2. Herbaceous without secondary growth. -

Spore Dispersal of Selaginella Denticulata, S. Helvetica, and S
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2020 Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae Schneller, Jakob ; Kessler, Michael DOI: https://doi.org/10.1640/0002-8444-110.2.58 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-187856 Journal Article Published Version Originally published at: Schneller, Jakob; Kessler, Michael (2020). Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae. American Fern Journal, 110(2):58-65. DOI: https://doi.org/10.1640/0002-8444-110.2.58 Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae Authors: Schneller, Jakob, and Kessler, Michael Source: American Fern Journal, 110(2) : 58-65 Published By: The American Fern Society URL: https://doi.org/10.1640/0002-8444-110.2.58 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non - commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. -

Phyton Annales Rei Botanicae
©Verlag Ferdinand Berger & Söhne Ges.m.b.H., Horn, Austria, download unter www.biologiezentrum.at PHYTON ANNALES REI BOTANICAE VOL. 45, FASC. 1 PAG. 1--144 30. 6. 2005 Phyton (Horn, Austria) Vol. 45 Fasc. 1 1-8 30. 6. 2005 The Gametophyte-Sporophyte Junction in Selaginella martensii SPRING (Selaginellales, Lycopodiophyta) By Hartmut H. HILGER*), Nancy KAPUSKAR**) and Wolfgang FREY*) With 6 Figures Received August 20, 2004 Key words: Lycopodiophyta, Pteridophyta, Selaginella, Selaginellales. - Anatomy;omy,, gametophyte-sporophytgame e junction, placental space. - Electron microscopy. Summary HILGER H. H., KAPUSKAR N. & FREY W. 2005. The gametophyte-sporophyte junction in Selaginella martensii SPRING (Selaginellales, Lycopodiophyta). - Phyton (Horn, Austria) 45 (1): 1-8, with 6 figures. - English with German summary. The gametophyte-sporophyte junction in Selaginella martensii (Selaginellales, Lycopodiophyta) consists of a sporophytic conical foot embedded in the maternal gametophytic tissue. Both generations are separated by a narrow placental space *) Prof. Dr. H. H. HILGER, Prof. Dr. W. FREY, Institut für Biologie - Systematische Botanik und Pflanzengeographie - der Freien Universität Berlin, Altensteinstrae 6, D-14195 Berlin, Germany; e-mail: [email protected], [email protected] berlin.de **) Dr. N. KAPUSKAR, Institut für Spezielle Botanik und Botanischer Garten der Johannes Gutenberg-Universität, Bentzelweg 2, D-55099 Mainz; e-mail: [email protected] ©Verlag Ferdinand Berger & Söhne Ges.m.b.H., Horn, Austria, download unter www.biologiezentrum.at filled with thin-walled collapsed cells of gametophytic origin. Gametophytic and sporophytic placental cells lack wall ingrowths, respectively transfer cells. Neither interdigitation nor intermingling of placental cells nor nacreous thickenings are de- veloped. The structure of the gametophyte-sporophyte junction in Selaginella mar- tensii resembles that of Isoetes boliviensis, the second lycopodiopside investigated, and thus differs from the junction described for pteridopsides and lycopods. -

Plastid Genomes of the Early Vascular Plant Genus Selaginella Have Unusual Direct Repeat Structures and Drastically Reduced Gene Numbers
International Journal of Molecular Sciences Article Plastid Genomes of the Early Vascular Plant Genus Selaginella Have Unusual Direct Repeat Structures and Drastically Reduced Gene Numbers Hyeonah Shim 1, Hyeon Ju Lee 1, Junki Lee 1,2, Hyun-Oh Lee 1,2, Jong-Hwa Kim 3, Tae-Jin Yang 1,* and Nam-Soo Kim 4,* 1 Department of Agriculture, Forestry and Bioresources, Plant Genomics & Breeding Institute, Research Institute of Agriculture and Life Sciences, College of Agriculture & Life Sciences, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 08826, Korea; [email protected] (H.S.); [email protected] (H.J.L.); [email protected] (J.L.); [email protected] (H.-O.L.) 2 Phyzen Genomics Institute, Seongnam 13558, Korea 3 Department of Horticulture, Kangwon National University, Chuncheon 24341, Korea; [email protected] 4 Department of Molecular Bioscience, Kangwon National University, Chuncheon 24341, Korea * Correspondence: [email protected] (T.-J.Y.); [email protected] (N.-S.K.); Tel.: +82-2-880-4547 (T.-J.Y.); +82-33-250-6472 (N.-S.K.) Abstract: The early vascular plants in the genus Selaginella, which is the sole genus of the Selaginel- laceae family, have an important place in evolutionary history, along with ferns, as such plants are valuable resources for deciphering plant evolution. In this study, we sequenced and assembled the plastid genome (plastome) sequences of two Selaginella tamariscina individuals, as well as Se- laginella stauntoniana and Selaginella involvens. Unlike the inverted repeat (IR) structures typically found in plant plastomes, Selaginella species had direct repeat (DR) structures, which were confirmed by Oxford Nanopore long-read sequence assembly. -

Sustainable Sourcing : Markets for Certified Chinese
SUSTAINABLE SOURCING: MARKETS FOR CERTIFIED CHINESE MEDICINAL AND AROMATIC PLANTS In collaboration with SUSTAINABLE SOURCING: MARKETS FOR CERTIFIED CHINESE MEDICINAL AND AROMATIC PLANTS SUSTAINABLE SOURCING: MARKETS FOR CERTIFIED CHINESE MEDICINAL AND AROMATIC PLANTS Abstract for trade information services ID=43163 2016 SITC-292.4 SUS International Trade Centre (ITC) Sustainable Sourcing: Markets for Certified Chinese Medicinal and Aromatic Plants. Geneva: ITC, 2016. xvi, 141 pages (Technical paper) Doc. No. SC-2016-5.E This study on the market potential of sustainably wild-collected botanical ingredients originating from the People’s Republic of China with fair and organic certifications provides an overview of current export trade in both wild-collected and cultivated botanical, algal and fungal ingredients from China, market segments such as the fair trade and organic sectors, and the market trends for certified ingredients. It also investigates which international standards would be the most appropriate and applicable to the special case of China in consideration of its biodiversity conservation efforts in traditional wild collection communities and regions, and includes bibliographical references (pp. 139–140). Descriptors: Medicinal Plants, Spices, Certification, Organic Products, Fair Trade, China, Market Research English For further information on this technical paper, contact Mr. Alexander Kasterine ([email protected]) The International Trade Centre (ITC) is the joint agency of the World Trade Organization and the United Nations. ITC, Palais des Nations, 1211 Geneva 10, Switzerland (www.intracen.org) Suggested citation: International Trade Centre (2016). Sustainable Sourcing: Markets for Certified Chinese Medicinal and Aromatic Plants, International Trade Centre, Geneva, Switzerland. This publication has been produced with the financial assistance of the European Union. -

Biogeographical Patterns of Species Richness, Range Size And
Biogeographical patterns of species richness, range size and phylogenetic diversity of ferns along elevational-latitudinal gradients in the tropics and its transition zone Kumulative Dissertation zur Erlangung als Doktorgrades der Naturwissenschaften (Dr.rer.nat.) dem Fachbereich Geographie der Philipps-Universität Marburg vorgelegt von Adriana Carolina Hernández Rojas aus Xalapa, Veracruz, Mexiko Marburg/Lahn, September 2020 Vom Fachbereich Geographie der Philipps-Universität Marburg als Dissertation am 10.09.2020 angenommen. Erstgutachter: Prof. Dr. Georg Miehe (Marburg) Zweitgutachterin: Prof. Dr. Maaike Bader (Marburg) Tag der mündlichen Prüfung: 27.10.2020 “An overwhelming body of evidence supports the conclusion that every organism alive today and all those who have ever lived are members of a shared heritage that extends back to the origin of life 3.8 billion years ago”. This sentence is an invitation to reflect about our non- independence as a living beins. We are part of something bigger! "Eine überwältigende Anzahl von Beweisen stützt die Schlussfolgerung, dass jeder heute lebende Organismus und alle, die jemals gelebt haben, Mitglieder eines gemeinsamen Erbes sind, das bis zum Ursprung des Lebens vor 3,8 Milliarden Jahren zurückreicht." Dieser Satz ist eine Einladung, über unsere Nichtunabhängigkeit als Lebende Wesen zu reflektieren. Wir sind Teil von etwas Größerem! PREFACE All doors were opened to start this travel, beginning for the many magical pristine forest of Ecuador, Sierra de Juárez Oaxaca and los Tuxtlas in Veracruz, some of the most biodiverse zones in the planet, were I had the honor to put my feet, contemplate their beauty and perfection and work in their mystical forest. It was a dream into reality! The collaboration with the German counterpart started at the beginning of my academic career and I never imagine that this will be continued to bring this research that summarizes the efforts of many researchers that worked hardly in the overwhelming and incredible biodiverse tropics. -

Natural Products from Genus Selaginella (Selaginellaceae)
ISSN: 2087-3948 (print) Vol. 3, No. 1, Pp.: 44-58 ISSN: 2087-3956 (electronic) March 2011 Review: Natural products from Genus Selaginella (Selaginellaceae) AHMAD DWI SETYAWAN♥ Department of Biology, Faculty of Mathematics and Natural Sciences, Sebelas Maret University, Surakarta 57126. Jl. Ir. Sutami 36A Surakarta 57126, Tel./fax. +62-271-663375, email: [email protected] Manuscript received: 28 Augustus 2010. Revision accepted: 4 October 2010. Abstract. Setyawan AD. 2011. Natural products from Genus Selaginella (Selaginellaceae). Nusantara Bioscience 3: 44-58. Selaginella is a potent medicinal-stuff, which contains diverse of natural products such as alkaloid, phenolic (flavonoid), and terpenoid. This species is traditionally used to cure several diseases especially for wound, after childbirth, and menstrual disorder. Biflavonoid, a dimeric form of flavonoids, is the most valuable natural products of Selaginella, which constituted at least 13 compounds, namely amentoflavone, 2',8''-biapigenin, delicaflavone, ginkgetin, heveaflavone, hinokiflavone, isocryptomerin, kayaflavone, ochnaflavone, podocarpusflavone A, robustaflavone, sumaflavone, and taiwaniaflavone. Ecologically, plants use biflavonoid to response environmental condition such as defense against pests, diseases, herbivory, and competitions; while human medically use biflavonoid especially for antioxidant, anti- inflammatory, and anti carcinogenic. Selaginella also contains valuable disaccharide, namely trehalose that has long been known for protecting from desiccation and allows surviving severe environmental stress. The compound has very prospects as molecular stabilizer in the industries based bioresources. Key words: natural products, biflavonoid, trehalose, Selaginella. Abstrak. Setyawan AD. 2011. Bahan alam dari Genus Selaginella (Selaginellaceae). Nusantara Bioscience 3: 44-58. Selaginella adalah bahan baku obat yang potensial, yang mengandung beragam metabolit sekunder seperti alkaloid, fenolik (flavonoid), dan terpenoid. -

Inventarisasi Selaginellaceae Di Kawasan Taman Wisata Alam Sicike-Cike Kabupaten Dairi Sumatera Utara
INVENTARISASI SELAGINELLACEAE DI KAWASAN TAMAN WISATA ALAM SICIKE-CIKE KABUPATEN DAIRI SUMATERA UTARA SKRIPSI OLEH: AFRIZAL AZALI 13.870.0022 FAKULTAS BIOLOGI UNIVERSITAS MEDAN AREA MEDAN 2017 UNIVERSITAS MEDAN AREA INVENTARISASI SELAGINELLACEAE DI KAWASAN TAMAN WISATA ALAM SICIKE-CIKE KABUPATEN DAIRI SUMATERA UTARA SKRIPSI OLEH: AFRIZAL AZALI 13.870.0022 Skripsi ini Sebagai Syarat Untuk Memperoleh Gelar Sarjana Sains Di Fakultas Biologi Universitas Medan Area FAKULTAS BIOLOGI UNIVERSITAS MEDAN AREA MEDAN 2017 UNIVERSITAS MEDAN AREA UNIVERSITAS MEDAN AREA UNIVERSITAS MEDAN AREA UNIVERSITAS MEDAN AREA ABSTRACT Natural Park (TWA) Sicike-Cike is highland tropical rain forest located in Kabupaten Dairi, North Sumatera. The park is home of various ferns. The purpose of this research is to inventory fern’s species classified as Selaginellaceae, in the above Park. Samples were obtained using “purposive sampling’(descriptive method) by exploration technique, there were 5 Specias identified; Selaginella intermedia, Selaginella longiaristata, Selaginella ornata, Selaginella plana, and Selaginella willdenowii. Keyword: Inventory, Selaginellaceae, TWA Sicike-Cike, Fern allies i UNIVERSITAS MEDAN AREA ABSTRAK Taman Wisata Alam (TWA) Sicike-cike adalah suatu Kawasan hutan hujan tropis dataran tinggi yang berlokasi di Kabupaten Dairi, Sumatera Utara. Didalamnya banyak terdapat bermacam-macam tumbuhan paku.. Penelitian ini bertujuan inventarisasi jenis-jenis tumbuhan paku yang tergolong dalam family Selaginellaceae di kawasan tersebut. Pengambilan sampel -

Lycopodiaceae) Weston Testo University of Vermont
University of Vermont ScholarWorks @ UVM Graduate College Dissertations and Theses Dissertations and Theses 2018 Devonian origin and Cenozoic radiation in the clubmosses (Lycopodiaceae) Weston Testo University of Vermont Follow this and additional works at: https://scholarworks.uvm.edu/graddis Part of the Systems Biology Commons Recommended Citation Testo, Weston, "Devonian origin and Cenozoic radiation in the clubmosses (Lycopodiaceae)" (2018). Graduate College Dissertations and Theses. 838. https://scholarworks.uvm.edu/graddis/838 This Dissertation is brought to you for free and open access by the Dissertations and Theses at ScholarWorks @ UVM. It has been accepted for inclusion in Graduate College Dissertations and Theses by an authorized administrator of ScholarWorks @ UVM. For more information, please contact [email protected]. DEVONIAN ORIGIN AND CENOZOIC RADIATION IN THE CLUBMOSSES (LYCOPODIACEAE) A Dissertation Presented by Weston Testo to The Faculty of the Graduate College of The University of Vermont In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Specializing in Plant Biology January, 2018 Defense Date: November 13, 2017 Dissertation Examination Committee: David S. Barrington, Ph.D., Advisor Ingi Agnarsson, Ph.D., Chairperson Jill Preston, Ph.D. Cathy Paris, Ph.D. Cynthia J. Forehand, Ph.D., Dean of the Graduate College ABSTRACT Together with the heterosporous lycophytes, the clubmoss family (Lycopodiaceae) is the sister lineage to all other vascular land plants. Given the family’s important position in the land-plant phylogeny, studying the evolutionary history of this group is an important step towards a better understanding of plant evolution. Despite this, little is known about the Lycopodiaceae, and a well-sampled, robust phylogeny of the group is lacking.