Phyton Annales Rei Botanicae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WRA Species Report
Family: Selaginellaceae Taxon: Selaginella braunii Synonym: Lycopodioides braunii (Baker) Kuntze Common Name: arborvitae fern Selaginella braunii fo. hieronymi Alderw. Braun's spike-moss Selaginella hieronymi Alderw. Chinese lace-fern spike-moss Selaginella vogelii Mett. treelet spike-moss Questionaire : current 20090513 Assessor: Assessor Designation: H(HPWRA) Status: Assessor Approved Data Entry Person: Assessor WRA Score 7 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- Low substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 y 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 n 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 y 301 Naturalized beyond native range y = 1*multiplier (see y Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see n Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see n Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see n Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see y Appendix 2) 401 Produces spines, thorns or -

Selaginellaceae: Traditional Use, Phytochemistry and Pharmacology
MS Editions BOLETIN LATINOAMERICANO Y DEL CARIBE DE PLANTAS MEDICINALES Y AROMÁTICAS 19 (3): 247 - 288 (2020) © / ISSN 0717 7917 / www.blacpma.ms-editions.cl Revisión | Review Selaginellaceae: traditional use, phytochemistry and pharmacology [Selaginellaceae: uso tradicional, fitoquímica y farmacología] Fernanda Priscila Santos Reginaldo, Isabelly Cristina de Matos Costa & Raquel Brandt Giordani College of Pharmacy, Pharmacy Department. University of Rio Grande do Norte, Natal, RN, Brazil. Contactos | Contacts: Raquel Brandt GIORDANI - E-mail address: [email protected] Abstract: Selaginella is the only genus from Selaginellaceae, and it is considered a key factor in studying evolution. The family managed to survive the many biotic and abiotic pressures during the last 400 million years. The purpose of this review is to provide an up-to-date overview of Selaginella in order to recognize their potential and evaluate future research opportunities. Carbohydrates, pigments, steroids, phenolic derivatives, mainly flavonoids, and alkaloids are the main natural products in Selaginella. A wide spectrum of in vitro and in vivo pharmacological activities, some of them pointed out by folk medicine, has been reported. Future studies should afford valuable new data on better explore the biological potential of the flavonoid amentoflavone and their derivatives as chemical bioactive entities; develop studies about toxicity and, finally, concentrate efforts on elucidate mechanisms of action for biological properties already reported. Keywords: Selaginella; Natural Products; Overview. Resumen: Selaginella es el único género de Selaginellaceae, y se considera un factor clave en el estudio de la evolución. La familia logró sobrevivir a las muchas presiones bióticas y abióticas durante los últimos 400 millones de años. -

Chapter 23: the Early Tracheophytes
Chapter 23 The Early Tracheophytes THE LYCOPHYTES Lycopodium Has a Homosporous Life Cycle Selaginella Has a Heterosporous Life Cycle Heterospory Allows for Greater Parental Investment Isoetes May Be the Only Living Member of the Lepidodendrid Group THE MONILOPHYTES Whisk Ferns Ophioglossalean Ferns Horsetails Marattialean Ferns True Ferns True Fern Sporophytes Typically Have Underground Stems Sexual Reproduction Usually Is Homosporous Fern Have a Variety of Alternative Means of Reproduction Ferns Have Ecological and Economic Importance SUMMARY PLANTS, PEOPLE, AND THE ENVIRONMENT: Sporophyte Prominence and Survival on Land PLANTS, PEOPLE, AND THE ENVIRONMENT: Coal, Smog, and Forest Decline THE OCCUPATION OF THE LAND PLANTS, PEOPLE, AND THE The First Tracheophytes Were ENVIRONMENT: Diversity Among the Ferns Rhyniophytes Tracheophytes Became Increasingly Better PLANTS, PEOPLE, AND THE Adapted to the Terrestrial Environment ENVIRONMENT: Fern Spores Relationships among Early Tracheophytes 1 KEY CONCEPTS 1. Tracheophytes, also called vascular plants, possess lignified water-conducting tissue (xylem). Approximately 14,000 species of tracheophytes reproduce by releasing spores and do not make seeds. These are sometimes called seedless vascular plants. Tracheophytes differ from bryophytes in possessing branched sporophytes that are dominant in the life cycle. These sporophytes are more tolerant of life on dry land than those of bryophytes because water movement is controlled by strongly lignified vascular tissue, stomata, and an extensive cuticle. The gametophytes, however still require a seasonally wet habitat, and water outside the plant is essential for the movement of sperm from antheridia to archegonia. 2. The rhyniophytes were the first tracheophytes. They consisted of dichotomously branching axes, lacking roots and leaves. They are all extinct. -
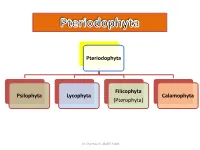
Pteriodophyta Psilophyta Lycophyta Filicophyta (Pterophyta) Calamophyta
Pteriodophyta Filicophyta Psilophyta Lycophyta Calamophyta (Pterophyta) Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora (Eligulatae) Order: Lycopodiales Family: Lycopodiaceae e.g. Lycopodium Subclass(2): Heterospora (Ligulatae) Order: Selaginellales Family: Selaginellaceae e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Phylum: Lepidophyta • It is characterized by: 1. The plant is differentiated into stem, leaves and roots. 2. Leaves are microphyllous (small, one vein and with no leaf gap). 3. The stele is protostele, siphonostele or polystele. 4. Protoxylem is exarch (ranging from a complete external cylinder to a polyarch stele in which there are many protoxylem ribs). 5. Sporangia are solitary, carried on special leaves (sporophylls). Sporophylls are usually collected in strobili. Dr. Shaimaa N. Abd El-Fatah . Among this phylum there are two independent evolutionary lines Heterospora (Ligulatae) Homospora (Eligulatae) • Characterized by presence • Eligulate (ligule lacking). of ligule (small outgrowth on • Homosporous (one type of the upper surface of the spores). leaf). • Heterosporous character (2 types of spores: microspores-- small, give rise to male gametophyte. & megaspores– larger, give rise to female gametophyte.) Dr. Shaimaa N. Abd El-Fatah • The phylum includes one class: Lycopodinae. • The class is classified into 4 orders: • Plants belong to the first order are homosporae (eligulatae), while the remaining three are heterosporae (ligulatae). Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora Subclass(2): Heterospora (Eligulatae) (Ligulatae) Order: Lycopodiales Order: Selaginellales Family: Lycopodiaceae Family: Selaginellaceae e.g. Lycopodium e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Lycopodiales This order is characterized by: 1. Homosporous and eligulatae. 2. Herbaceous without secondary growth. -

Spore Dispersal of Selaginella Denticulata, S. Helvetica, and S
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2020 Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae Schneller, Jakob ; Kessler, Michael DOI: https://doi.org/10.1640/0002-8444-110.2.58 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-187856 Journal Article Published Version Originally published at: Schneller, Jakob; Kessler, Michael (2020). Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae. American Fern Journal, 110(2):58-65. DOI: https://doi.org/10.1640/0002-8444-110.2.58 Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae Authors: Schneller, Jakob, and Kessler, Michael Source: American Fern Journal, 110(2) : 58-65 Published By: The American Fern Society URL: https://doi.org/10.1640/0002-8444-110.2.58 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non - commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. -

Selaginella Wangpeishanii (Selaginellaceae), a New Lycophyte from a Limestone Cave in Guizhou, China
Phytotaxa 164 (3): 195–199 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ Article PHYTOTAXA Copyright © 2014 Magnolia Press ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.164.3.5 Selaginella wangpeishanii (Selaginellaceae), a new lycophyte from a limestone cave in Guizhou, China LI-BING ZHANG1 , QING-WEN SUN2 & HAI HE3* 1Chengdu Institute of Biology, Chinese Academy of Sciences, P.O. Box 416, Chengdu, Sichuan 610041, P. R. China and Missouri Botanical Garden, P.O. Box 299, St. Louis, Missouri 63166-0299, U.S.A. 2Department of Pharmacy, Guiyang College of Traditional Chinese Medicine, Guiyang, Guizhou 550002, P. R. China 3College of Life Sciences, Chongqing Normal University, Shapingba, Chongqing 400047, P. R. China *Author for correspondence: e-mail: [email protected] Abstract Selaginella wangpeishanii, a new species of the lycophyte genus Selaginella (Selaginellaceae) from a limestone cave in southern Guizhou, China is described and illustrated. The new species is different from any species in the genus known so far in China by having some ultimate branches bearing microphylls in ascending order of trophophylls, sporophylls or sporophyll-like microphylls, and trophophylls (TST arrangement of microphylls). Morphologically, S. wangpeishanii is most similar to S. gebaueriana, but is distinct by smaller plants and serrulate margins of lateral trophophylls. Selaginella wangpeishanii is currently known only from a single population and is considered to be Critically Endangered (CR), based on IUCN Red List criteria. Key words: Selaginella wangpeishanii, Guizhou, TST arrangement of microphylls, IUCN Red List Introduction Selaginella Beauvois (1804: 478) (Selaginellaceae) is the largest lycophyte genus with about 750 species mainly distributed in tropical and subtropical areas (Jermy 1990). -

Natural Products from Genus Selaginella (Selaginellaceae)
ISSN: 2087-3948 (print) Vol. 3, No. 1, Pp.: 44-58 ISSN: 2087-3956 (electronic) March 2011 Review: Natural products from Genus Selaginella (Selaginellaceae) AHMAD DWI SETYAWAN♥ Department of Biology, Faculty of Mathematics and Natural Sciences, Sebelas Maret University, Surakarta 57126. Jl. Ir. Sutami 36A Surakarta 57126, Tel./fax. +62-271-663375, email: [email protected] Manuscript received: 28 Augustus 2010. Revision accepted: 4 October 2010. Abstract. Setyawan AD. 2011. Natural products from Genus Selaginella (Selaginellaceae). Nusantara Bioscience 3: 44-58. Selaginella is a potent medicinal-stuff, which contains diverse of natural products such as alkaloid, phenolic (flavonoid), and terpenoid. This species is traditionally used to cure several diseases especially for wound, after childbirth, and menstrual disorder. Biflavonoid, a dimeric form of flavonoids, is the most valuable natural products of Selaginella, which constituted at least 13 compounds, namely amentoflavone, 2',8''-biapigenin, delicaflavone, ginkgetin, heveaflavone, hinokiflavone, isocryptomerin, kayaflavone, ochnaflavone, podocarpusflavone A, robustaflavone, sumaflavone, and taiwaniaflavone. Ecologically, plants use biflavonoid to response environmental condition such as defense against pests, diseases, herbivory, and competitions; while human medically use biflavonoid especially for antioxidant, anti- inflammatory, and anti carcinogenic. Selaginella also contains valuable disaccharide, namely trehalose that has long been known for protecting from desiccation and allows surviving severe environmental stress. The compound has very prospects as molecular stabilizer in the industries based bioresources. Key words: natural products, biflavonoid, trehalose, Selaginella. Abstrak. Setyawan AD. 2011. Bahan alam dari Genus Selaginella (Selaginellaceae). Nusantara Bioscience 3: 44-58. Selaginella adalah bahan baku obat yang potensial, yang mengandung beragam metabolit sekunder seperti alkaloid, fenolik (flavonoid), dan terpenoid. -

Diversity and Evolution of the Megaphyll in Euphyllophytes
G Model PALEVO-665; No. of Pages 16 ARTICLE IN PRESS C. R. Palevol xxx (2012) xxx–xxx Contents lists available at SciVerse ScienceDirect Comptes Rendus Palevol w ww.sciencedirect.com General palaeontology, systematics and evolution (Palaeobotany) Diversity and evolution of the megaphyll in Euphyllophytes: Phylogenetic hypotheses and the problem of foliar organ definition Diversité et évolution de la mégaphylle chez les Euphyllophytes : hypothèses phylogénétiques et le problème de la définition de l’organe foliaire ∗ Adèle Corvez , Véronique Barriel , Jean-Yves Dubuisson UMR 7207 CNRS-MNHN-UPMC, centre de recherches en paléobiodiversité et paléoenvironnements, 57, rue Cuvier, CP 48, 75005 Paris, France a r t i c l e i n f o a b s t r a c t Article history: Recent paleobotanical studies suggest that megaphylls evolved several times in land plant st Received 1 February 2012 evolution, implying that behind the single word “megaphyll” are hidden very differ- Accepted after revision 23 May 2012 ent notions and concepts. We therefore review current knowledge about diverse foliar Available online xxx organs and related characters observed in fossil and living plants, using one phylogenetic hypothesis to infer their origins and evolution. Four foliar organs and one lateral axis are Presented by Philippe Taquet described in detail and differ by the different combination of four main characters: lateral organ symmetry, abdaxity, planation and webbing. Phylogenetic analyses show that the Keywords: “true” megaphyll appeared at least twice in Euphyllophytes, and that the history of the Euphyllophytes Megaphyll four main characters is different in each case. The current definition of the megaphyll is questioned; we propose a clear and accurate terminology in order to remove ambiguities Bilateral symmetry Abdaxity of the current vocabulary. -

Phytoliths of Pteridophytes
South African Journal of Botany 77 (2011) 10–19 Minireview Phytoliths of pteridophytes J. Mazumdar UGC Centre for Advanced Study, Department of Botany, The University of Burdwan, Burdwan-713104, India Received 3 June 2010; received in revised form 14 July 2010; accepted 28 July 2010 Abstract Study of phytoliths of pteridophytes is an emerging area of research. Literature on this aspect is limited but increasing. Some recent findings have shown that phytoliths may have systematic and phylogenetic utility in pteridophytes. Phytoliths are functionally significant for the development and survival of pteridophytes. Experiments with some pteridophytes have revealed various aspects of silica uptake, deposition and biological effects. © 2010 SAAB. Published by Elsevier B.V. All rights reserved. Keywords: Biogenic silica; Ferns; Pteridophytes; Phytolith; Silicification 1. Introduction In spite of environmental effects on silica uptake, ongoing investigations indicate that phytolith formation is primarily Many plants deposit silica as solid hydrated Silicone dioxide under genetic control (Piperno, 2006). Phytoliths have been (SiO2,nH2O) in the cell lumen or in intercellular spaces, where used successfully as taxonomic tools in angiosperms, especially it is known as “phytoliths” or “plant stones” (Greek, phyto = in monocots (Piperno, 1988; Tubb et al., 1993). Less plant, lithos = stone) or “silicophytoliths” or “opal phytoliths” or information is available about pteridophytic phytoliths. The “plant opal” or “opaline silica” or “biogenic silica” or “bioliths”. objective of this review is to evaluate the present status and The term “phytolith” may also be applied to other mineral future prospects of phytolith research in pteridophytes. structures of plant origin, including calcium oxalate crystals, but is more usually restricted to silica particles (Prychid et al., 2004). -

Megaphylls, Microphylls and the Evolution of Leaf Development
Opinion Megaphylls, microphylls and the evolution of leaf development Alexandru M.F. Tomescu Department of Biological Sciences, Humboldt State University, Arcata, CA 95521, USA Originally coined to emphasize morphological differ- However, the microphyll–megaphyll divide is not as ences, ‘microphyll’ and ‘megaphyll’ became synon- clear cut, and morphological definitions that contrast ymous with the idea that vascular plant leaves are not microphylls and megaphylls as mutually exclusive con- homologous. Although it is now accepted that leaves cepts of leaves are inconsistent. Moreover, current under- evolved independently in several euphyllophyte standing of plant phylogeny and leaf development fails to lineages, ‘megaphyll’ has grown to reflect another type shed light on the origin of microphylls, and supports of homology, that of euphyllophyte leaf precursor struc- several independent origins of megaphylls. Here, I review tures. However, evidence from the fossil record and plant phylogeny and developmental data from fossil and developmental pathways fails to indicate homology extant trachephytes, as well as the current understanding and suggests homoplasy of precursor structures. Thus, of genetic pathways controlling leaf development, to argue as I discuss here, ‘megaphyll’ should be abandoned that the megaphyll concept should be abandoned because because it perpetuates an unsupported idea of it perpetuates misconceptions and confusion based on homology, leading to misconceptions that pervade plant unsupported homology. biology thinking and can bias hypothesis and inference in developmental and phylogenetic studies. Alternative Morphological inconsistencies and overlap in the definitions are needed that are based on development microphyll–megaphyll dichotomy and phylogeny for different independently evolved leaf Microphylls are defined as leaves of small size, with simple types. -

1455-Rev.Pdf
RESEARCH ARTICLE Gigantic chloroplasts, including bizonoplasts, are common in shade-adapted species of the ancient vascular plant family Selaginellaceae Jian-Wei Liu1, Shau-Fu Li1, Chin-Ting Wu2, Iván A. Valdespino3 , Jia-Fang Ho1,2, Yeh-Hua Wu1,2, Ho-Ming Chang4 , Te-Yu Guu1, Mei-Fang Kao5, Clive Chesson6, Sauren Das7 , Hank Oppenheimer8, Ane Bakutis9, Peter Saenger10, Noris Salazar Allen11 , Jean W. H. Yong12 , Bayu Adjie13, Ruth Kiew14, Nalini Nadkarni15 , Chun-Lin Huang16 , Peter Chesson1,17 , and Chiou-Rong Sheue1,17,18 Manuscript received 11 August 2019; revision accepted 21 January 2020. PREMISE: Unique among vascular plants, some species of Selaginella have single giant 1 Department of Life Sciences & Research Center for Global Change chloroplasts in their epidermal or upper mesophyll cells (monoplastidy, M), varying in Biology, National Chung Hsing University, Taichung, Taiwan structure between species. Structural variants include several forms of bizonoplast with 2 Department of Biological Resources, National Chiayi University, unique dimorphic ultrastructure. Better understanding of these structural variants, their Chiayi, Taiwan prevalence, environmental correlates and phylogenetic association, has the potential to 3 Departamento de Botánica, Facultad de Ciencias Naturales, shed new light on chloroplast biology unavailable from any other plant group. Exactas y Tecnología, Universidad de Panamá; Sistema Nacional de Investigación (SNI), SENACYT, Panama, Panama METHODS: The chloroplast ultrastructure of 76 Selaginella species -

PTERIDOPHYTIC FLORA of INDIA (August 2020- March 2023)
PTERIDOPHYTIC FLORA OF INDIA (August 2020- March 2023) . A. BENNIAMIN, B.S.KHOLIA, V.K.RAWAT , BRIJESH KUMAR D. JESUBALAN PTERIDOPHYTE DIVERSITY IN INDIA • TOTAL NO OF SPECIES : 1107 spp. • NO OF FAMILIES : 34 • NO OF GENERA : 130 (Fraser jenkins et al., 2016) DR. A. BENNIAMIAN, WRC, PUNE Sr.No Name of the Family Total No of species 1 Selaginellaceae 11 (New) 2 Marattiaceae 5 3 Osmundaceae 6 4 Plagiogyraceae 4 5 Dipteriaceae 1 6 Dryopteridaceae 204 7 Nephrolepidaceae 9 8 Oleandraceae 3 9 Lomariopsidaceae 28 10 Vittariaceae 6(New) 11 Blechnaceae 11 12 Azollaceae 3 13 Salviniaceae 3 14 Glechniaceae 7 15 Lygodiaceae 9 16 Schizaeaceae 3 17 Marsileaceae 3 Total Ca.316spp DR. B.S. KHOLIA, NRC, DEHRADUN Sr. No Name of Family Total No 1. Lycopodiaceae 28 2. Selaginellaceae 17 3. Isoetaceae 4 4. Equisetaceae 4 5. Psilotaceae 2 6. Cyatheaceae 11 7. Lindsaeaceae 20 8. Hymenophyllaceae 36 9. Dennstaedtiaceae(with SC) 27 10. Aspleniaceae 32 11. Vittariaceae 10 13. Thelypteridaceae 83 14. Davalliaceae 19(New) Total Ca.274spp DR.V.K. RAWAT, APRC, ITANAGAR Name of Family Total 1. Selaginellaceae 19 2. Pteridaceae (except Adiantum) 155 Ophioglossaceae 20 3. 4. Polypodiaceae (except 110 Lepisorus) Total 304spp. DR. BRIJESH KUMAR, CRC, ALLAHABAD Name of Family/Genera No of species 1. Lepisorus 20 (Polypodiaceae) 2. Woodsiaceae 130 3. Aspleniaceae 87 4. Adiantum (Pteridaceae) 20 Total 257spp. YEAR WISE BREAK UP NAME OF Total no of 1styear 2nd year 3rd year SCIENTIST species 2020-2021 2021-2022 2022-2023 Dr. A. BENNIAMIN 316spp. 110spp. 110spp. 106spp. Dr. B.S.KHOLIA 274spp.