1455-Rev.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WRA Species Report
Family: Selaginellaceae Taxon: Selaginella braunii Synonym: Lycopodioides braunii (Baker) Kuntze Common Name: arborvitae fern Selaginella braunii fo. hieronymi Alderw. Braun's spike-moss Selaginella hieronymi Alderw. Chinese lace-fern spike-moss Selaginella vogelii Mett. treelet spike-moss Questionaire : current 20090513 Assessor: Assessor Designation: H(HPWRA) Status: Assessor Approved Data Entry Person: Assessor WRA Score 7 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- Low substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 y 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 n 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 y 301 Naturalized beyond native range y = 1*multiplier (see y Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see n Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see n Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see n Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see y Appendix 2) 401 Produces spines, thorns or -

Selaginellaceae: Traditional Use, Phytochemistry and Pharmacology
MS Editions BOLETIN LATINOAMERICANO Y DEL CARIBE DE PLANTAS MEDICINALES Y AROMÁTICAS 19 (3): 247 - 288 (2020) © / ISSN 0717 7917 / www.blacpma.ms-editions.cl Revisión | Review Selaginellaceae: traditional use, phytochemistry and pharmacology [Selaginellaceae: uso tradicional, fitoquímica y farmacología] Fernanda Priscila Santos Reginaldo, Isabelly Cristina de Matos Costa & Raquel Brandt Giordani College of Pharmacy, Pharmacy Department. University of Rio Grande do Norte, Natal, RN, Brazil. Contactos | Contacts: Raquel Brandt GIORDANI - E-mail address: [email protected] Abstract: Selaginella is the only genus from Selaginellaceae, and it is considered a key factor in studying evolution. The family managed to survive the many biotic and abiotic pressures during the last 400 million years. The purpose of this review is to provide an up-to-date overview of Selaginella in order to recognize their potential and evaluate future research opportunities. Carbohydrates, pigments, steroids, phenolic derivatives, mainly flavonoids, and alkaloids are the main natural products in Selaginella. A wide spectrum of in vitro and in vivo pharmacological activities, some of them pointed out by folk medicine, has been reported. Future studies should afford valuable new data on better explore the biological potential of the flavonoid amentoflavone and their derivatives as chemical bioactive entities; develop studies about toxicity and, finally, concentrate efforts on elucidate mechanisms of action for biological properties already reported. Keywords: Selaginella; Natural Products; Overview. Resumen: Selaginella es el único género de Selaginellaceae, y se considera un factor clave en el estudio de la evolución. La familia logró sobrevivir a las muchas presiones bióticas y abióticas durante los últimos 400 millones de años. -

The Origin of Alternation of Generations in Land Plants
Theoriginof alternation of generations inlandplants: afocuson matrotrophy andhexose transport Linda K.E.Graham and LeeW .Wilcox Department of Botany,University of Wisconsin, 430Lincoln Drive, Madison,WI 53706, USA (lkgraham@facsta¡.wisc .edu ) Alifehistory involving alternation of two developmentally associated, multicellular generations (sporophyteand gametophyte) is anautapomorphy of embryophytes (bryophytes + vascularplants) . Microfossil dataindicate that Mid ^Late Ordovicianland plants possessed such alifecycle, and that the originof alternationof generationspreceded this date.Molecular phylogenetic data unambiguously relate charophyceangreen algae to the ancestryof monophyletic embryophytes, and identify bryophytes as early-divergentland plants. Comparison of reproduction in charophyceans and bryophytes suggests that the followingstages occurredduring evolutionary origin of embryophytic alternation of generations: (i) originof oogamy;(ii) retention ofeggsand zygotes on the parentalthallus; (iii) originof matrotrophy (regulatedtransfer ofnutritional and morphogenetic solutes fromparental cells tothe nextgeneration); (iv)origin of a multicellularsporophyte generation ;and(v) origin of non-£ agellate, walled spores. Oogamy,egg/zygoteretention andmatrotrophy characterize at least some moderncharophyceans, and arepostulated to represent pre-adaptativefeatures inherited byembryophytes from ancestral charophyceans.Matrotrophy is hypothesizedto have preceded originof the multicellularsporophytes of plants,and to represent acritical innovation.Molecular -

Chapter 23: the Early Tracheophytes
Chapter 23 The Early Tracheophytes THE LYCOPHYTES Lycopodium Has a Homosporous Life Cycle Selaginella Has a Heterosporous Life Cycle Heterospory Allows for Greater Parental Investment Isoetes May Be the Only Living Member of the Lepidodendrid Group THE MONILOPHYTES Whisk Ferns Ophioglossalean Ferns Horsetails Marattialean Ferns True Ferns True Fern Sporophytes Typically Have Underground Stems Sexual Reproduction Usually Is Homosporous Fern Have a Variety of Alternative Means of Reproduction Ferns Have Ecological and Economic Importance SUMMARY PLANTS, PEOPLE, AND THE ENVIRONMENT: Sporophyte Prominence and Survival on Land PLANTS, PEOPLE, AND THE ENVIRONMENT: Coal, Smog, and Forest Decline THE OCCUPATION OF THE LAND PLANTS, PEOPLE, AND THE The First Tracheophytes Were ENVIRONMENT: Diversity Among the Ferns Rhyniophytes Tracheophytes Became Increasingly Better PLANTS, PEOPLE, AND THE Adapted to the Terrestrial Environment ENVIRONMENT: Fern Spores Relationships among Early Tracheophytes 1 KEY CONCEPTS 1. Tracheophytes, also called vascular plants, possess lignified water-conducting tissue (xylem). Approximately 14,000 species of tracheophytes reproduce by releasing spores and do not make seeds. These are sometimes called seedless vascular plants. Tracheophytes differ from bryophytes in possessing branched sporophytes that are dominant in the life cycle. These sporophytes are more tolerant of life on dry land than those of bryophytes because water movement is controlled by strongly lignified vascular tissue, stomata, and an extensive cuticle. The gametophytes, however still require a seasonally wet habitat, and water outside the plant is essential for the movement of sperm from antheridia to archegonia. 2. The rhyniophytes were the first tracheophytes. They consisted of dichotomously branching axes, lacking roots and leaves. They are all extinct. -

Jurnal Ilmu-Ilmu Hayati
P-ISSN 0126-1754 E-ISSN 2337-8751 Terakreditasi 200/M/KPT/2020 Volume 20 Nomor 1, April 2021 Jurnal Ilmu-ilmu Hayati Berita Biologi Vol. 20 No. 1 Hlm. 1 – 145 Bogor, April 2021 ISSN 0126-1754 Pusat Penelitian Biologi - LIPI BERITA BIOLOGI Vol. 20 No. 1 April 2021 Terakreditasi Berdasarkan Keputusan Direktur Jendral Penguatan Riset dan Pengembangan, Kemenristekdikti RI 200/M/KPT/2020 Tim Redaksi (Editorial Team) Andria Agusta (Pemimpin Redaksi, Editor in Chief) (Kimia Bahan Alam, Pusat Penelitian Kimia - LIPI) Kartika Dewi (Redaksi Pelaksana, Managing Editor) (Taksonomi Nematoda, Pusat Penelitian Biologi - LIPI) Kusumadewi Sri Yulita (Sistematika Molekuler Tumbuhan, Pusat Penelitian Biologi - LIPI) Gono Semiadi (Mammalogi, Pusat Penelitian Biologi - LIPI) Atit Kanti (Mikrobiologi, Pusat Penelitian Biologi - LIPI) Siti Sundari (Ekologi Lingkungan, Pusat Penelitian Biologi - LIPI) Arif Nurkanto (Mikrobiologi, Pusat Penelitian Biologi - LIPI) Kartika Dewi (Taksonomi Nematoda, Pusat Penelitian Biologi - LIPI) Dwi Setyo Rini (Biologi Molekuler Tumbuhan, Pusat Penelitian Biologi - LIPI) Desain dan Layout (Design and Layout) Liana Astuti Kesekretariatan (Secretary) Nira Ariasari Z Alamat (Address) Pusat Penelitian Biologi-LIPI Kompleks Cibinong Science Center (CSC-LIPI) Jalan Raya Jakarta-Bogor KM 46, Cibinong 16911, Bogor-Indonesia Telepon (021) 8765066 - 8765067 Faksimili (021) 8765059 Email: [email protected] [email protected] [email protected] Keterangan foto cover depan: Efek Cekaman Kromium Terhadap Profil Mikro-anatomi Cabai (Capsicum annuum L.) (Notes of cover picture): Micro-anatomical of the stems structure of 400x magnification. (Halaman 107) P-ISSN 0126-1754 E-ISSN 2337-8751 Terakreditasi 200/M/KPT/2020 Volume 20 Nomor 1, April 2021 Jurnal Ilmu-ilmu Hayati Berita Biologi Vol. -

Cell Wall Ribosomes Nucleus Chloroplast Cytoplasm
Cell Wall Ribosomes Nucleus Nickname: Protector Nickname: Protein Maker Nickname: Brain The cell wall is the outer covering of a Plant cell. It is Ribosomes read the recipe from the The nucleus is the largest organelle in a cell. The a strong and stiff and made of DNA and use this recipe to make nucleus directs all activity in the cell. It also controls cellulose. It supports and protects the plant cell by proteins. The nucleus tells the the growth and reproduction of the cell. holding it upright. It ribosomes which proteins to make. In humans, the nucleus contains 46 chromosomes allows water, oxygen and carbon dioxide to pass in out They are found in both plant and which are the instructions for all the activities in your of plant cell. animal cells. In a cell they can be found cell and body. floating around in the cytoplasm or attached to the endoplasmic reticulum. Chloroplast Cytoplasm Endoplasmic Reticulum Nickname: Oven Nickname: Gel Nickname: Highway Chloroplasts are oval structures that that contain a green Cytoplasm is the gel like fluid inside a The endoplasmic reticulum (ER) is the transportation pigment called chlorophyll. This allows plants to make cell. The organelles are floating around in center for the cell. The ER is like the conveyor belt, you their own food through the process of photosynthesis. this fluid. would see at a supermarket, except instead of moving your groceries it moves proteins from one part of the cell Chloroplasts are necessary for photosynthesis, the food to another. The Endoplasmic Reticulum looks like a making process, to occur. -

Download PDF ( 838KB )
Gardens’ Bulletin Singapore 67(2): 297–303. 2015 297 doi: 10.3850/S2382581215000253 Studies on Begonia (Begoniaceae) of the Moluccas II: a new species from Seram, Indonesia W.H. Ardi1 & D.C. Thomas2 1Bogor Botanic Gardens, Jl. Ir. H. Juanda No. 13, Bogor, Indonesia [email protected] 2Herbarium, Singapore Botanic Gardens, National Parks Board, 1 Cluny Road, Singapore 259569 [email protected] ABSTRACT. A new species of Begonia L., Begonia galeolepis Ardi & D.C.Thomas, is described from Seram, Maluku province, Indonesia. The species is endemic to Seram and belongs to Begonia section Petermannia. An identification key to the seven Begonia species known from the Moluccas is provided. Keywords. Begonia galeolepis, Maluku Islands, Manusela National Park Introduction Our understanding of the Begonia L. flora of the Moluccas (also known as the Maluku Islands), an archipelago within Indonesia located between the islands of Sulawesi and New Guinea, is very limited because of the paucity of herbarium collections from the region and a lack of alpha-taxonomic baseline work on eastern Malesian Begonia. The Begonia Resource Centre (Hughes et al., 2015) comprises records of only 138 Moluccan Begonia specimens, most of which are not identified to species level, and the rest belonging to six species (Ardi et al., 2014; see identification key to the Moluccan species below). Recent expeditions by Bali Botanic Garden, and a joint expedition between Bogor Botanic Gardens and Fairchild Tropical Botanic Gardens to major islands of the Moluccas (Halmahera, Seram and Ternate), have brought to light material of several Begonia species that do not conform with any species previously reported from the archipelago. -
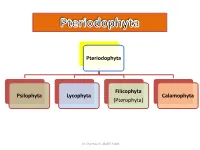
Pteriodophyta Psilophyta Lycophyta Filicophyta (Pterophyta) Calamophyta
Pteriodophyta Filicophyta Psilophyta Lycophyta Calamophyta (Pterophyta) Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora (Eligulatae) Order: Lycopodiales Family: Lycopodiaceae e.g. Lycopodium Subclass(2): Heterospora (Ligulatae) Order: Selaginellales Family: Selaginellaceae e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Phylum: Lepidophyta • It is characterized by: 1. The plant is differentiated into stem, leaves and roots. 2. Leaves are microphyllous (small, one vein and with no leaf gap). 3. The stele is protostele, siphonostele or polystele. 4. Protoxylem is exarch (ranging from a complete external cylinder to a polyarch stele in which there are many protoxylem ribs). 5. Sporangia are solitary, carried on special leaves (sporophylls). Sporophylls are usually collected in strobili. Dr. Shaimaa N. Abd El-Fatah . Among this phylum there are two independent evolutionary lines Heterospora (Ligulatae) Homospora (Eligulatae) • Characterized by presence • Eligulate (ligule lacking). of ligule (small outgrowth on • Homosporous (one type of the upper surface of the spores). leaf). • Heterosporous character (2 types of spores: microspores-- small, give rise to male gametophyte. & megaspores– larger, give rise to female gametophyte.) Dr. Shaimaa N. Abd El-Fatah • The phylum includes one class: Lycopodinae. • The class is classified into 4 orders: • Plants belong to the first order are homosporae (eligulatae), while the remaining three are heterosporae (ligulatae). Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora Subclass(2): Heterospora (Eligulatae) (Ligulatae) Order: Lycopodiales Order: Selaginellales Family: Lycopodiaceae Family: Selaginellaceae e.g. Lycopodium e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Lycopodiales This order is characterized by: 1. Homosporous and eligulatae. 2. Herbaceous without secondary growth. -

Brown Algae and 4) the Oomycetes (Water Molds)
Protista Classification Excavata The kingdom Protista (in the five kingdom system) contains mostly unicellular eukaryotes. This taxonomic grouping is polyphyletic and based only Alveolates on cellular structure and life styles not on any molecular evidence. Using molecular biology and detailed comparison of cell structure, scientists are now beginning to see evolutionary SAR Stramenopila history in the protists. The ongoing changes in the protest phylogeny are rapidly changing with each new piece of evidence. The following classification suggests 4 “supergroups” within the Rhizaria original Protista kingdom and the taxonomy is still being worked out. This lab is looking at one current hypothesis shown on the right. Some of the organisms are grouped together because Archaeplastida of very strong support and others are controversial. It is important to focus on the characteristics of each clade which explains why they are grouped together. This lab will only look at the groups that Amoebozoans were once included in the Protista kingdom and the other groups (higher plants, fungi, and animals) will be Unikonta examined in future labs. Opisthokonts Protista Classification Excavata Starting with the four “Supergroups”, we will divide the rest into different levels called clades. A Clade is defined as a group of Alveolates biological taxa (as species) that includes all descendants of one common ancestor. Too simplify this process, we have included a cladogram we will be using throughout the SAR Stramenopila course. We will divide or expand parts of the cladogram to emphasize evolutionary relationships. For the protists, we will divide Rhizaria the supergroups into smaller clades assigning them artificial numbers (clade1, clade2, clade3) to establish a grouping at a specific level. -

(SSC) Region of Chloroplast Genomes1
NEWS & VIEWS AMERICAN JOURNAL OF BOTANY LETTER TO THE EDITOR Sources of inversion variation in the small single copy (SSC) region of chloroplast genomes1 Joseph F. Walker 2 , Robert K. Jansen 3,4 , Michael J. Zanis 5 , and Nancy C. Emery6,7 Modern sequencing technology has led to a proliferation of whole- Walker et al., 2014 ; Zhang et al., 2014 ; Wang et al., 2015 ). Th ese genome sequences of chloroplasts in a growing number of plant analyses compare the SSC orientation among lineages using a single lineages, bringing opportunities for comparisons that provide in- plastome to represent each lineage and thus have missed the within- sights into the evolutionary history of the plastomes and their host individual variation that exists in this region. Currently, whole- plants ( Jansen et al., 2007 ; Doorduin et al., 2011 ). Amid the emerg- chloroplast genomes are published in GenBank without preference ing literature in this area is a hypothesis that the small single copy for the orientation of the SSC region, leading to apparent variation (SSC) region is a “hotspot” for inversion events (sensu Liu et al., in the orientation of the SSC region among individuals that is actu- 2013 ) because diff erent orientations of the region have been re- ally due to chloroplast heteroplasmy within individuals ( Wolfe and ported in relatively high frequencies among closely related taxa Randle, 2004 ), as originally described by Palmer (1983) . For exam- ( Liu et al., 2013 ; Walker et al., 2014 ). We would like to draw atten- ple, two sequences of Lactuca sativa that have been independently tion to a study by Palmer (1983) that bears heavily on this discus- published (NC_007578 and DQ_383816) were entered with diff er- sion, yet has been overlooked by several authors of publications ent orientations of the SSC region, which could be interpreted as investigating whole-chloroplast genome sequence order, including a major inversion existing within the species if the investigators are one study by some of the authors of this letter ( Walker et al., 2014 ). -

Spore Dispersal of Selaginella Denticulata, S. Helvetica, and S
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2020 Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae Schneller, Jakob ; Kessler, Michael DOI: https://doi.org/10.1640/0002-8444-110.2.58 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-187856 Journal Article Published Version Originally published at: Schneller, Jakob; Kessler, Michael (2020). Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae. American Fern Journal, 110(2):58-65. DOI: https://doi.org/10.1640/0002-8444-110.2.58 Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae Authors: Schneller, Jakob, and Kessler, Michael Source: American Fern Journal, 110(2) : 58-65 Published By: The American Fern Society URL: https://doi.org/10.1640/0002-8444-110.2.58 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non - commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. -

The Structure and Function of Plastids
The Structure and Function of Plastids Edited by Robert R. Wise University of Wisconsin, Oshkosh WI, USA and J. Kenneth Hoober Arizona State University, Tempe AZ, USA Contents From the Series Editor v Contents xi Preface xix A Dedication to Pioneers of Research on Chloroplast Structure xxi Color Plates xxxiii Section I Plastid Origin and Development 1 The Diversity of Plastid Form and Function 3–25 Robert R. Wise Summary 3 I. Introduction 4 II. The Plastid Family 5 III. Chloroplasts and their Specializations 13 IV. Concluding Remarks 20 Acknowledgements 21 References 21 2 Chloroplast Development: Whence and Whither 27–51 J. Kenneth Hoober Summary 27 I. Introduction 28 II. Brief Review of Plastid Evolution 28 III. Development of the Chloroplast 32 IV. Overview of Photosynthesis 43 References 46 3 Protein Import Into Chloroplasts: Who, When, and How? 53–74 Ute C. Vothknecht and J¨urgen Soll Summary 53 I. Introduction 54 II. On the Road to the Chloroplast 56 III. Protein Translocation via Toc and Tic 58 IV. Variations on Toc and Tic Translocation 63 V. Protein Translocation and Chloroplast Biogenesis 64 VI. The Evolutionary Origin of Toc and Tic 66 VII. Intraplastidal Transport 66 VIII. Protein Translocation into Complex Plastids 69 References 70 xi 4 Origin and Evolution of Plastids: Genomic View on the Unification and Diversity of Plastids 75–102 Naoki Sato Summary 76 I. Introduction: Unification and Diversity 76 II. Endosymbiotic Origin of Plastids: The Major Unifying Principle 78 III. Origin and Evolution of Plastid Diversity 85 IV. Conclusion: Opposing Principles in the Evolution of Plastids 97 Acknowledgements 98 References 98 5 The Mechanism of Plastid Division: The Structure and Origin of The Plastid Division Apparatus 103–121 Shin-ya Miyagishima and Tsuneyoshi Kuroiwa Summary 104 I.