ABSTRACT Unexpected Environmental Conditions Suggest
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lycopodium (Spp) L. Ground Pine
Kasey Hartz Natural Area Reference Sheet Lycopodium (spp) L. Ground pine. Lycopodiaceae (Club-moss Family) Blooming season: Not a flowering plant. Spores produced in July-September. Plant: Tree like in form, 10-25 cm tall. Horizontal stem, not a true rhizome; grows at one end only, and the opposite end dies, resulting in the plant slowly moving forward year by year (Billington 1952). Leaves: Evergreen, scale like. Flower: None, spores are produced on strobilus cones. Fruit: None, but what is formed are strobilus cones which are sessile, having no stalks. Usually 1-3 cones, 1.5-5.5 cm long, maturing July-September. Spores are sulphur yellow with a high oil content. Can be confused with: Many species of Lycopodium can be confused and hybrids may also be found. Geographic range: Type specimen location: State: Throughout. Regional: Newfoundland - Alaska, south to North Carolina and Indiana. Habitat: Local: Riparian. Regional: Moist woods. Common local companions: Pine, ferns, maples, and beech Kasey Hartz Natural Area Reference Sheet Lycopodium obscurum L. 2 Ground Pine Usages: Human: Because of the spores have a high oil content and are quite flammable, they were used as flash powder for the first photographic cameras(Harris 2003); for fireworks (Billington 1952); and to imitate lightning flashes for theatrical performances (Millspaugh 1892, 1974). Their oil content led to them being used by pharmacists in boxes of pills to prevent them sticking together (possibly a different species of Lycopodium). Medicinal uses in the past included treatments for gout, menstrual disorders, nervous disorders, fevers, and as a styptic and an emetic. -

RI Equisetopsida and Lycopodiopsida.Indd
IIntroductionntroduction byby FFrancisrancis UnderwoodUnderwood Rhode Island Equisetopsida, Lycopodiopsida and Isoetopsida Special Th anks to the following for giving permission for the use their images. Robbin Moran New York Botanical Garden George Yatskievych and Ann Larson Missouri Botanical Garden Jan De Laet, plantsystematics.org Th is pdf is a companion publication to Rhode Island Equisetopsida, Lycopodiopsida & Isoetopsida at among-ri-wildfl owers.org Th e Elfi n Press 2016 Introduction Formerly known as fern allies, Horsetails, Club-mosses, Fir-mosses, Spike-mosses and Quillworts are plants that have an alternate generation life-cycle similar to ferns, having both sporophyte and gametophyte stages. Equisetopsida Horsetails date from the Devonian period (416 to 359 million years ago) in earth’s history where they were trees up to 110 feet in height and helped to form the coal deposits of the Carboniferous period. Only one genus has survived to modern times (Equisetum). Horsetails Horsetails (Equisetum) have jointed stems with whorls of thin narrow leaves. In the sporophyte stage, they have a sterile and fertile form. Th ey produce only one type of spore. While the gametophytes produced from the spores appear to be plentiful, the successful reproduction of the sporophyte form is low with most Horsetails reproducing vegetatively. Lycopodiopsida Lycopodiopsida includes the clubmosses (Dendrolycopodium, Diphasiastrum, Lycopodiella, Lycopodium , Spinulum) and Fir-mosses (Huperzia) Clubmosses Clubmosses are evergreen plants that produce only microspores that develop into a gametophyte capable of producing both sperm and egg cells. Club-mosses can produce the spores either in leaf axils or at the top of their stems. Th e spore capsules form in a cone-like structures (strobili) at the top of the plants. -

WRA Species Report
Family: Selaginellaceae Taxon: Selaginella braunii Synonym: Lycopodioides braunii (Baker) Kuntze Common Name: arborvitae fern Selaginella braunii fo. hieronymi Alderw. Braun's spike-moss Selaginella hieronymi Alderw. Chinese lace-fern spike-moss Selaginella vogelii Mett. treelet spike-moss Questionaire : current 20090513 Assessor: Assessor Designation: H(HPWRA) Status: Assessor Approved Data Entry Person: Assessor WRA Score 7 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- Low substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 y 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 n 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 y 301 Naturalized beyond native range y = 1*multiplier (see y Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see n Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see n Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see n Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see y Appendix 2) 401 Produces spines, thorns or -

Effects of Lycopodium Clavatum and Equisetum Arvense Extracts from Western Romania
Romanian Biotechnological Letters Vol. , No. x, Copyright © 2016 University of Bucharest Printed in Romania. All rights reserved ORIGINAL PAPER Effects of Lycopodium clavatum and equisetum arvense extracts from western Romania Received for publication, July, 07, 2014 Accepted, October, 13, 2015 MARIA SUCIU1, FELIX AUREL MIC1, LUCIAN BARBU-TUDORAN2, VASILE MUNTEAN2, ALEXANDRA TEODORA GRUIA3,* 1University of Medicine and Pharmacy “Victor Babes”, Department of Functional Sciences, Timisoara, 2, Eftimie Murgu Sq., Timisoara, 300041, Timis County, Romania 2Babes-Bolyai University, Biology and Geology Department, 5-7 Clinicilor Str., Cluj-Napoca, 400084, Cluj County, Romania. 3Emergency Clinical County Hospital Timisoara, Regional Centre for Transplant Immunology Department, 10, Iosif Bulbuca Blvd., Timisoara, 300736, Timis County, Romania. *Address for correspondence to: [email protected], 10, Iosif Bulbuca Blvd., Timisoara, 300736, Timis County, Romania. Abbreviations: ALT–alanin transaminases, AST–aspartate transaminases, GC-MS–gas chromatograph coupled with mass spectrometry. Abstract Plants have always excited interest because of their active principles that could be a source of healing in various affections. The aim of this study was to demonstrate that the hepatoprotective and antimicrobial effects of Lycopodium clavatum and Equisetum arvense from the Western parts of Romania (Arad County) are not as pronounced as described in literature, against xenobiotic intoxication or microbial infection. To identify the plants active compounds, -

Selaginellaceae: Traditional Use, Phytochemistry and Pharmacology
MS Editions BOLETIN LATINOAMERICANO Y DEL CARIBE DE PLANTAS MEDICINALES Y AROMÁTICAS 19 (3): 247 - 288 (2020) © / ISSN 0717 7917 / www.blacpma.ms-editions.cl Revisión | Review Selaginellaceae: traditional use, phytochemistry and pharmacology [Selaginellaceae: uso tradicional, fitoquímica y farmacología] Fernanda Priscila Santos Reginaldo, Isabelly Cristina de Matos Costa & Raquel Brandt Giordani College of Pharmacy, Pharmacy Department. University of Rio Grande do Norte, Natal, RN, Brazil. Contactos | Contacts: Raquel Brandt GIORDANI - E-mail address: [email protected] Abstract: Selaginella is the only genus from Selaginellaceae, and it is considered a key factor in studying evolution. The family managed to survive the many biotic and abiotic pressures during the last 400 million years. The purpose of this review is to provide an up-to-date overview of Selaginella in order to recognize their potential and evaluate future research opportunities. Carbohydrates, pigments, steroids, phenolic derivatives, mainly flavonoids, and alkaloids are the main natural products in Selaginella. A wide spectrum of in vitro and in vivo pharmacological activities, some of them pointed out by folk medicine, has been reported. Future studies should afford valuable new data on better explore the biological potential of the flavonoid amentoflavone and their derivatives as chemical bioactive entities; develop studies about toxicity and, finally, concentrate efforts on elucidate mechanisms of action for biological properties already reported. Keywords: Selaginella; Natural Products; Overview. Resumen: Selaginella es el único género de Selaginellaceae, y se considera un factor clave en el estudio de la evolución. La familia logró sobrevivir a las muchas presiones bióticas y abióticas durante los últimos 400 millones de años. -

Notes on Some Species of Diphasiastrum
Preslia, Praha, 47: 232 - 240, 1975 Notes on some species of Diphasiastrum Poznamky k n~kterym druhum rodu Dipha11iaatrum Josef Holub HOLUB J. (1975): Notes on some species of Diphasiastrum. - Preslia, Praha, 47: 232- 240. Taxonomic and nomenclatural problems of some species of Diphasiastrum HOLUB are discussed. A special attention is pa.id to the interspecies D. / X / issleri and D. / x / zei leri. Original plants of D. / x / issleri correspond to the combination D. alpinum - D. complanatum. Plants corresponding to the combination D. alpinum - D. tristachyum have been collected in the ~umava Mts. Some taxa described from the subarctic regions of Europe and North America are shown to belong most probably to the neglected interspecies D. / x / zeileri. Botanical I nstitute, Czechoslovak Academy of Sciences, 25~ 43 Prithonice, Czecho.,lovakia. INTRODUCTION This is a second part of my study of the new genus Diphasiastrum (HOLUB 1975), which could not be published in this journal in its entirety. Notes on taxonomy and nomenclature are selected from materials gathered originally for my "Catalogue of Czechoslovak vascular plants". With regard to the character of that work the present observations summarize the results of my own studies and suggests problems to be studied in the future. OBSERVATIONS f!.iphasiastrum alpinum (L.) HOLUB Two varieties have been described in this species (both under the name Lycopodium alpi nttm L.): var. thellungii HERTER from Switzerland and var. planiramulosum TAKEDA from Japan. Both these taxa, especially the first one, require a taxonomic revision; the possibility cannot be exclu<led that they are conspecific with D. / x / issleri. -

Chapter 23: the Early Tracheophytes
Chapter 23 The Early Tracheophytes THE LYCOPHYTES Lycopodium Has a Homosporous Life Cycle Selaginella Has a Heterosporous Life Cycle Heterospory Allows for Greater Parental Investment Isoetes May Be the Only Living Member of the Lepidodendrid Group THE MONILOPHYTES Whisk Ferns Ophioglossalean Ferns Horsetails Marattialean Ferns True Ferns True Fern Sporophytes Typically Have Underground Stems Sexual Reproduction Usually Is Homosporous Fern Have a Variety of Alternative Means of Reproduction Ferns Have Ecological and Economic Importance SUMMARY PLANTS, PEOPLE, AND THE ENVIRONMENT: Sporophyte Prominence and Survival on Land PLANTS, PEOPLE, AND THE ENVIRONMENT: Coal, Smog, and Forest Decline THE OCCUPATION OF THE LAND PLANTS, PEOPLE, AND THE The First Tracheophytes Were ENVIRONMENT: Diversity Among the Ferns Rhyniophytes Tracheophytes Became Increasingly Better PLANTS, PEOPLE, AND THE Adapted to the Terrestrial Environment ENVIRONMENT: Fern Spores Relationships among Early Tracheophytes 1 KEY CONCEPTS 1. Tracheophytes, also called vascular plants, possess lignified water-conducting tissue (xylem). Approximately 14,000 species of tracheophytes reproduce by releasing spores and do not make seeds. These are sometimes called seedless vascular plants. Tracheophytes differ from bryophytes in possessing branched sporophytes that are dominant in the life cycle. These sporophytes are more tolerant of life on dry land than those of bryophytes because water movement is controlled by strongly lignified vascular tissue, stomata, and an extensive cuticle. The gametophytes, however still require a seasonally wet habitat, and water outside the plant is essential for the movement of sperm from antheridia to archegonia. 2. The rhyniophytes were the first tracheophytes. They consisted of dichotomously branching axes, lacking roots and leaves. They are all extinct. -

Ecophysiology of Four Co-Occurring Lycophyte Species: an Investigation of Functional Convergence
Research Article Ecophysiology of four co-occurring lycophyte species: an investigation of functional convergence Jacqlynn Zier, Bryce Belanger, Genevieve Trahan and James E. Watkins* Department of Biology, Colgate University, Hamilton, NY 13346, USA Received: 22 June 2015; Accepted: 7 November 2015; Published: 24 November 2015 Associate Editor: Tim J. Brodribb Citation: Zier J, Belanger B, Trahan G, Watkins JE. 2015. Ecophysiology of four co-occurring lycophyte species: an investigation of functional convergence. AoB PLANTS 7: plv137; doi:10.1093/aobpla/plv137 Abstract. Lycophytes are the most early divergent extant lineage of vascular land plants. The group has a broad global distribution ranging from tundra to tropical forests and can make up an important component of temperate northeast US forests. We know very little about the in situ ecophysiology of this group and apparently no study has eval- uated if lycophytes conform to functional patterns expected by the leaf economics spectrum hypothesis. To determine factors influencing photosynthetic capacity (Amax), we analysed several physiological traits related to photosynthesis to include stomatal, nutrient, vascular traits, and patterns of biomass distribution in four coexisting temperate lycophyte species: Lycopodium clavatum, Spinulum annotinum, Diphasiastrum digitatum and Dendrolycopodium dendroi- deum. We found no difference in maximum photosynthetic rates across species, yet wide variation in other traits. We also found that Amax was not related to leaf nitrogen concentration and is more tied to stomatal conductance, suggestive of a fundamentally different sets of constraints on photosynthesis in these lycophyte taxa compared with ferns and seed plants. These findings complement the hydropassive model of stomatal control in lycophytes and may reflect canaliza- tion of function in this group. -
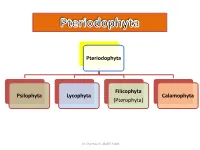
Pteriodophyta Psilophyta Lycophyta Filicophyta (Pterophyta) Calamophyta
Pteriodophyta Filicophyta Psilophyta Lycophyta Calamophyta (Pterophyta) Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora (Eligulatae) Order: Lycopodiales Family: Lycopodiaceae e.g. Lycopodium Subclass(2): Heterospora (Ligulatae) Order: Selaginellales Family: Selaginellaceae e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Phylum: Lepidophyta • It is characterized by: 1. The plant is differentiated into stem, leaves and roots. 2. Leaves are microphyllous (small, one vein and with no leaf gap). 3. The stele is protostele, siphonostele or polystele. 4. Protoxylem is exarch (ranging from a complete external cylinder to a polyarch stele in which there are many protoxylem ribs). 5. Sporangia are solitary, carried on special leaves (sporophylls). Sporophylls are usually collected in strobili. Dr. Shaimaa N. Abd El-Fatah . Among this phylum there are two independent evolutionary lines Heterospora (Ligulatae) Homospora (Eligulatae) • Characterized by presence • Eligulate (ligule lacking). of ligule (small outgrowth on • Homosporous (one type of the upper surface of the spores). leaf). • Heterosporous character (2 types of spores: microspores-- small, give rise to male gametophyte. & megaspores– larger, give rise to female gametophyte.) Dr. Shaimaa N. Abd El-Fatah • The phylum includes one class: Lycopodinae. • The class is classified into 4 orders: • Plants belong to the first order are homosporae (eligulatae), while the remaining three are heterosporae (ligulatae). Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora Subclass(2): Heterospora (Eligulatae) (Ligulatae) Order: Lycopodiales Order: Selaginellales Family: Lycopodiaceae Family: Selaginellaceae e.g. Lycopodium e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Lycopodiales This order is characterized by: 1. Homosporous and eligulatae. 2. Herbaceous without secondary growth. -

Spore Dispersal of Selaginella Denticulata, S. Helvetica, and S
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2020 Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae Schneller, Jakob ; Kessler, Michael DOI: https://doi.org/10.1640/0002-8444-110.2.58 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-187856 Journal Article Published Version Originally published at: Schneller, Jakob; Kessler, Michael (2020). Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae. American Fern Journal, 110(2):58-65. DOI: https://doi.org/10.1640/0002-8444-110.2.58 Spore dispersal of Selaginella denticulata, S. helvetica, and S. selaginoides, and the significance of heterospory in Selaginellacae Authors: Schneller, Jakob, and Kessler, Michael Source: American Fern Journal, 110(2) : 58-65 Published By: The American Fern Society URL: https://doi.org/10.1640/0002-8444-110.2.58 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non - commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. -

Conservation Assessment for Groundcedar and Stiff Clubmoss In
United States Department of Agriculture Conservation Assessment Forest Service for Groundcedar and Stiff Rocky Mountain Region Clubmoss in the Black Black Hills National Forest Hills National Forest South Custer, South Dakota Dakota and Wyoming March 2003 J.Hope Hornbeck, Deanna J. Reyher, Carolyn Sieg and Reed W. Crook Species Assessment of Groundcedar and Stiff Clubmoss in the Black Hills National Forest, South Dakota and Wyoming J. Hope Hornbeck, Deanna J. Reyher, Carolyn Hull Sieg and Reed W. Crook J. Hope Hornbeck is a Botanist with the Black Hills National Forest in Custer, South Dakota. She completed a B.S. in Environmental Biology at The University of Montana and a M.S. in Plant Biology at the University of Minnesota. Deanna J. Reyher is an Ecologist/Soil Scientist with the Black Hills National Forest in Custer, South Dakota. She completed a B.S. degree in Agronomy from the University of Nebraska. Carolyn Hull Sieg is a Research Plant Ecologist with the Rocky Mountain Research Station in Flagstaff, Arizona. She completed a B.S. in Wildlife Biology and M.S. in Range Science from Colorado State University and a Ph.D. in Range and Wildlife Management at Texas Tech University. Reed W. Crook is a Botanist with the Black Hills National Forest in Custer, South Dakota. He completed a B.S. in Botany at Brigham Young University, and a M.S. in Plant Morphology and Ph.D. in Plant Systematics at the University of Georgia. EXECUTIVE SUMMARY Stiff clubmoss (Lycopodium annotinum L.) and groundcedar (Lycopodium complanatum L.; synonym = Diphasiastrum complanatum [L.] Holub.) (Lycopodiaceae) are circumboreal clubmoss species that are widely distributed in North American boreal habitats. -

Phyton Annales Rei Botanicae
©Verlag Ferdinand Berger & Söhne Ges.m.b.H., Horn, Austria, download unter www.biologiezentrum.at PHYTON ANNALES REI BOTANICAE VOL. 45, FASC. 1 PAG. 1--144 30. 6. 2005 Phyton (Horn, Austria) Vol. 45 Fasc. 1 1-8 30. 6. 2005 The Gametophyte-Sporophyte Junction in Selaginella martensii SPRING (Selaginellales, Lycopodiophyta) By Hartmut H. HILGER*), Nancy KAPUSKAR**) and Wolfgang FREY*) With 6 Figures Received August 20, 2004 Key words: Lycopodiophyta, Pteridophyta, Selaginella, Selaginellales. - Anatomy;omy,, gametophyte-sporophytgame e junction, placental space. - Electron microscopy. Summary HILGER H. H., KAPUSKAR N. & FREY W. 2005. The gametophyte-sporophyte junction in Selaginella martensii SPRING (Selaginellales, Lycopodiophyta). - Phyton (Horn, Austria) 45 (1): 1-8, with 6 figures. - English with German summary. The gametophyte-sporophyte junction in Selaginella martensii (Selaginellales, Lycopodiophyta) consists of a sporophytic conical foot embedded in the maternal gametophytic tissue. Both generations are separated by a narrow placental space *) Prof. Dr. H. H. HILGER, Prof. Dr. W. FREY, Institut für Biologie - Systematische Botanik und Pflanzengeographie - der Freien Universität Berlin, Altensteinstrae 6, D-14195 Berlin, Germany; e-mail: [email protected], [email protected] berlin.de **) Dr. N. KAPUSKAR, Institut für Spezielle Botanik und Botanischer Garten der Johannes Gutenberg-Universität, Bentzelweg 2, D-55099 Mainz; e-mail: [email protected] ©Verlag Ferdinand Berger & Söhne Ges.m.b.H., Horn, Austria, download unter www.biologiezentrum.at filled with thin-walled collapsed cells of gametophytic origin. Gametophytic and sporophytic placental cells lack wall ingrowths, respectively transfer cells. Neither interdigitation nor intermingling of placental cells nor nacreous thickenings are de- veloped. The structure of the gametophyte-sporophyte junction in Selaginella mar- tensii resembles that of Isoetes boliviensis, the second lycopodiopside investigated, and thus differs from the junction described for pteridopsides and lycopods.