Conservation Assessment for Groundcedar and Stiff Clubmoss In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lycopodium (Spp) L. Ground Pine
Kasey Hartz Natural Area Reference Sheet Lycopodium (spp) L. Ground pine. Lycopodiaceae (Club-moss Family) Blooming season: Not a flowering plant. Spores produced in July-September. Plant: Tree like in form, 10-25 cm tall. Horizontal stem, not a true rhizome; grows at one end only, and the opposite end dies, resulting in the plant slowly moving forward year by year (Billington 1952). Leaves: Evergreen, scale like. Flower: None, spores are produced on strobilus cones. Fruit: None, but what is formed are strobilus cones which are sessile, having no stalks. Usually 1-3 cones, 1.5-5.5 cm long, maturing July-September. Spores are sulphur yellow with a high oil content. Can be confused with: Many species of Lycopodium can be confused and hybrids may also be found. Geographic range: Type specimen location: State: Throughout. Regional: Newfoundland - Alaska, south to North Carolina and Indiana. Habitat: Local: Riparian. Regional: Moist woods. Common local companions: Pine, ferns, maples, and beech Kasey Hartz Natural Area Reference Sheet Lycopodium obscurum L. 2 Ground Pine Usages: Human: Because of the spores have a high oil content and are quite flammable, they were used as flash powder for the first photographic cameras(Harris 2003); for fireworks (Billington 1952); and to imitate lightning flashes for theatrical performances (Millspaugh 1892, 1974). Their oil content led to them being used by pharmacists in boxes of pills to prevent them sticking together (possibly a different species of Lycopodium). Medicinal uses in the past included treatments for gout, menstrual disorders, nervous disorders, fevers, and as a styptic and an emetic. -

RI Equisetopsida and Lycopodiopsida.Indd
IIntroductionntroduction byby FFrancisrancis UnderwoodUnderwood Rhode Island Equisetopsida, Lycopodiopsida and Isoetopsida Special Th anks to the following for giving permission for the use their images. Robbin Moran New York Botanical Garden George Yatskievych and Ann Larson Missouri Botanical Garden Jan De Laet, plantsystematics.org Th is pdf is a companion publication to Rhode Island Equisetopsida, Lycopodiopsida & Isoetopsida at among-ri-wildfl owers.org Th e Elfi n Press 2016 Introduction Formerly known as fern allies, Horsetails, Club-mosses, Fir-mosses, Spike-mosses and Quillworts are plants that have an alternate generation life-cycle similar to ferns, having both sporophyte and gametophyte stages. Equisetopsida Horsetails date from the Devonian period (416 to 359 million years ago) in earth’s history where they were trees up to 110 feet in height and helped to form the coal deposits of the Carboniferous period. Only one genus has survived to modern times (Equisetum). Horsetails Horsetails (Equisetum) have jointed stems with whorls of thin narrow leaves. In the sporophyte stage, they have a sterile and fertile form. Th ey produce only one type of spore. While the gametophytes produced from the spores appear to be plentiful, the successful reproduction of the sporophyte form is low with most Horsetails reproducing vegetatively. Lycopodiopsida Lycopodiopsida includes the clubmosses (Dendrolycopodium, Diphasiastrum, Lycopodiella, Lycopodium , Spinulum) and Fir-mosses (Huperzia) Clubmosses Clubmosses are evergreen plants that produce only microspores that develop into a gametophyte capable of producing both sperm and egg cells. Club-mosses can produce the spores either in leaf axils or at the top of their stems. Th e spore capsules form in a cone-like structures (strobili) at the top of the plants. -

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

Introduction to Botany. Lecture 29
Kingdom Vegetabilia: plants Introduction to Botany. Lecture 29 Alexey Shipunov Minot State University November 12th, 2010 Shipunov BIOL 154.29 Kingdom Vegetabilia: plants Outline 1 Kingdom Vegetabilia: plants Bryophyta: mosses Pteridophyta: ferns and allies Shipunov BIOL 154.29 Bryophyta: mosses Kingdom Vegetabilia: plants Pteridophyta: ferns and allies Life cycle of mosses (picture from the board) Shipunov BIOL 154.29 Bryophyta: mosses Kingdom Vegetabilia: plants Pteridophyta: ferns and allies Three main groups (subphyla) Hepaticae—liverworts. Three classes, most primitive are Haplomitriopsida. Body has dorsal and ventral parts, sporogon bag-like, without columella, spores with elaters. Bryophytina—true mosses. Six classes, most important are Sphagnopsida (peat mosses), Polytrichopsida (haircap mosses) and Bryopsida. Body radial, sporogon long, with columella, spores without elaters. Anthocerotophytina—hornworts. One class. Body flattened, sporogon long, green, with columella and stomata, spores with elaters. Shipunov BIOL 154.29 Bryophyta: mosses Kingdom Vegetabilia: plants Pteridophyta: ferns and allies Haplomitrium gibbsiae, primitive liverwort Shipunov BIOL 154.29 Bryophyta: mosses Kingdom Vegetabilia: plants Pteridophyta: ferns and allies Elaters of liverworts (Lepidozia sp.) Shipunov BIOL 154.29 Bryophyta: mosses Kingdom Vegetabilia: plants Pteridophyta: ferns and allies Sphagnum sp. (Bryophyta, Sphagnopsida) with sporogons Shipunov BIOL 154.29 Bryophyta: mosses Kingdom Vegetabilia: plants Pteridophyta: ferns and allies Dawsonia -

Download Document
African countries and neighbouring islands covered by the Synopsis. S T R E L I T Z I A 23 Synopsis of the Lycopodiophyta and Pteridophyta of Africa, Madagascar and neighbouring islands by J.P. Roux Pretoria 2009 S T R E L I T Z I A This series has replaced Memoirs of the Botanical Survey of South Africa and Annals of the Kirstenbosch Botanic Gardens which SANBI inherited from its predecessor organisations. The plant genus Strelitzia occurs naturally in the eastern parts of southern Africa. It comprises three arborescent species, known as wild bananas, and two acaulescent species, known as crane flowers or bird-of-paradise flowers. The logo of the South African National Biodiversity Institute is based on the striking inflorescence of Strelitzia reginae, a native of the Eastern Cape and KwaZulu-Natal that has become a garden favourite worldwide. It sym- bolises the commitment of the Institute to champion the exploration, conservation, sustain- able use, appreciation and enjoyment of South Africa’s exceptionally rich biodiversity for all people. J.P. Roux South African National Biodiversity Institute, Compton Herbarium, Cape Town SCIENTIFIC EDITOR: Gerrit Germishuizen TECHNICAL EDITOR: Emsie du Plessis DESIGN & LAYOUT: Elizma Fouché COVER DESIGN: Elizma Fouché, incorporating Blechnum palmiforme on Gough Island PHOTOGRAPHS J.P. Roux Citing this publication ROUX, J.P. 2009. Synopsis of the Lycopodiophyta and Pteridophyta of Africa, Madagascar and neighbouring islands. Strelitzia 23. South African National Biodiversity Institute, Pretoria. ISBN: 978-1-919976-48-8 © Published by: South African National Biodiversity Institute. Obtainable from: SANBI Bookshop, Private Bag X101, Pretoria, 0001 South Africa. -

Effects of Lycopodium Clavatum and Equisetum Arvense Extracts from Western Romania
Romanian Biotechnological Letters Vol. , No. x, Copyright © 2016 University of Bucharest Printed in Romania. All rights reserved ORIGINAL PAPER Effects of Lycopodium clavatum and equisetum arvense extracts from western Romania Received for publication, July, 07, 2014 Accepted, October, 13, 2015 MARIA SUCIU1, FELIX AUREL MIC1, LUCIAN BARBU-TUDORAN2, VASILE MUNTEAN2, ALEXANDRA TEODORA GRUIA3,* 1University of Medicine and Pharmacy “Victor Babes”, Department of Functional Sciences, Timisoara, 2, Eftimie Murgu Sq., Timisoara, 300041, Timis County, Romania 2Babes-Bolyai University, Biology and Geology Department, 5-7 Clinicilor Str., Cluj-Napoca, 400084, Cluj County, Romania. 3Emergency Clinical County Hospital Timisoara, Regional Centre for Transplant Immunology Department, 10, Iosif Bulbuca Blvd., Timisoara, 300736, Timis County, Romania. *Address for correspondence to: [email protected], 10, Iosif Bulbuca Blvd., Timisoara, 300736, Timis County, Romania. Abbreviations: ALT–alanin transaminases, AST–aspartate transaminases, GC-MS–gas chromatograph coupled with mass spectrometry. Abstract Plants have always excited interest because of their active principles that could be a source of healing in various affections. The aim of this study was to demonstrate that the hepatoprotective and antimicrobial effects of Lycopodium clavatum and Equisetum arvense from the Western parts of Romania (Arad County) are not as pronounced as described in literature, against xenobiotic intoxication or microbial infection. To identify the plants active compounds, -

263 Determining the Amount of Nucleic Acids in Medicinal
Analele Universităţii din Oradea, Fascicula Protecţia Mediului Vol. XXIII, 2014 DETERMINING THE AMOUNT OF NUCLEIC ACIDS IN MEDICINAL FERNS COLLECTED FROM DIFFERENT AREAS OF ARAD AND BIHOR COUNTY Pallag Annamaria*, Osser Gyongyi**, Orodan Maria**, Honiges Ana**, Gîtea Daniela*, Pașca Bianca* *University of Oradea, Faculty of Medicine and Pharmacy, Pharmacy Department, P-ta 1 Decembrie, no. 10, Oradea, 410223, Romania, [email protected] **Vasile Goldis Western University of Arad, Romania, College of Medicine, Pharmacy and Dentistry, Biophysics Department Abstract Lycopodium clavatum L. and Equisetum arvense L. are ferns, Pteridophytes, and are widely used medicinal plants. These species are not cultivated in Romania, the medicinal product coming from traditional collection centers, so the exact origin of the product is difficult to follow. In this work we are presenting the obtained results of determining the amount of nucleic acids in case of several Lycopodium clavatum and Equisetum arvense L. populations, in terms of the species’ homogeneity. There were studied the stems of Lycopodium clavatum L. and Equisetum arvense L., collected from different regions - Oradea’s area, Cefa area - of Bihor County, and from Macea Botanical Garden (MBG), Arad county in the period of April - September 2014. As a result of the study we have observed the presence of differences in the amount of nucleic acids between the studied populations. Keywords: Lycopodium clavatum L., Equisetum arvense L., nucleic acids INTRODUCTION Pteridophytes are known from as far back as the Silurian, or some 380 million years ago (Bennici, 2008, Gifford et al, 1988). The Pteridophytes occupy the intermediate position between the bryophytes and the phanerogams (Graham, 1993). -

Notes on Some Species of Diphasiastrum
Preslia, Praha, 47: 232 - 240, 1975 Notes on some species of Diphasiastrum Poznamky k n~kterym druhum rodu Dipha11iaatrum Josef Holub HOLUB J. (1975): Notes on some species of Diphasiastrum. - Preslia, Praha, 47: 232- 240. Taxonomic and nomenclatural problems of some species of Diphasiastrum HOLUB are discussed. A special attention is pa.id to the interspecies D. / X / issleri and D. / x / zei leri. Original plants of D. / x / issleri correspond to the combination D. alpinum - D. complanatum. Plants corresponding to the combination D. alpinum - D. tristachyum have been collected in the ~umava Mts. Some taxa described from the subarctic regions of Europe and North America are shown to belong most probably to the neglected interspecies D. / x / zeileri. Botanical I nstitute, Czechoslovak Academy of Sciences, 25~ 43 Prithonice, Czecho.,lovakia. INTRODUCTION This is a second part of my study of the new genus Diphasiastrum (HOLUB 1975), which could not be published in this journal in its entirety. Notes on taxonomy and nomenclature are selected from materials gathered originally for my "Catalogue of Czechoslovak vascular plants". With regard to the character of that work the present observations summarize the results of my own studies and suggests problems to be studied in the future. OBSERVATIONS f!.iphasiastrum alpinum (L.) HOLUB Two varieties have been described in this species (both under the name Lycopodium alpi nttm L.): var. thellungii HERTER from Switzerland and var. planiramulosum TAKEDA from Japan. Both these taxa, especially the first one, require a taxonomic revision; the possibility cannot be exclu<led that they are conspecific with D. / x / issleri. -
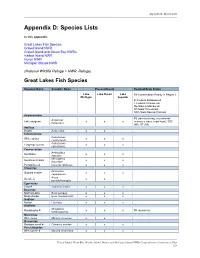
Species Lists
Appendix D: Species Lists Appendix D: Species Lists In this appendix: Great Lakes Fish Species Gravel Island NWR Gravel Island and Green Bay NWRs Harbor Island NWR Huron NWR Michigan Islands NWR (National Wildlife Refuge = NWR, Refuge) Great Lakes Fish Species Common Name Scientific Name Present/Absent Regional/State Status Lake Lake Huron Lake R3-Conservation Priority in Region 3 Michigan Superior E- Federal Endangered T-Federal Threatened SE-State Endangered ST-State Threatened SSC-State Special Concern Acipenseridae R3 (rare/declining, recreational/ Acipenser Lake sturgeon x x x economic value, tribal trust), SSC fulvescens (WI), ST (MI) Amiidae Bowfin Amia calva x x Catostomidae Catostomus White sucker x x x commersoni Catostomus Longnose sucker x x x catostomus Centrarchidae Ambloplites Rockbass x x x rupestris Micropterus Smallmouth bass x x x dolomieui Pumpkinseed Lepomis gibbosus x x x Clupeidae Dorosoma Gizzard shad # x x x cepedianum Alosa Alewife # x x pseudoharengus Cyprinidae Carp # Cyprinus Carpio x x x Esocidae Northern pike Esox Lucieus x x x Muskellunge Esox masquinongy x x x Gadidae Burbot Lota lota x x x Gobiidae Neogobius Round goby # x x x R3 (nuisance) melanostomus Moronidae White bass Morone chrysops x x Osmeridae Rainbow smelt # Osmerus mordax x x x Percichthyidae White perch # Morone americana x x x Gravel Island, Green Bay, Harbor Island, Huron, and Michigan Islands NWRs/Comprehensive Conservation Plan 221 Appendix D: Species Lists Common Name Scientific Name Present/Absent Regional/State Status Percidae R3 (rare/declining, -

Climate Change Vulnerability in Biodiversity Sector
Ministry of Envrionment and Physical Planning of the Republic of Macedonia United Nations Development Programme Project 00075206 “Third National Report to UNFCCC” Project report Climate change vulnerability in Biodiversity sector Melovski Ljupčo, Matevski Vlado, Hristovski Slavčo Institute of Biology, Faculty of Natural Sciences and Mathematics, Ss. Cyril and Methodius University, Skopje, Republic of Macedonia Skopje, 2013 2 Contents 1 National Circumstances related to climate change vulnerability in Biodiversity Sector ................ 5 1.1 Introduction – climate change and Biodiversity Sector ................................................................ 5 1.1.1 Climate change vulnerability in Biodiversity Sector........................................................ 5 1.1.2 Climate change adaptation in Biodiversity Sector .......................................................... 7 1.2 Overview of Biodiversity Sector .................................................................................................... 7 1.2.1 Characteristics ................................................................................................................. 7 1.2.2 Major stakeholders ....................................................................................................... 15 1.2.3 Sector documents ......................................................................................................... 17 1.2.4 Data availability ............................................................................................................ -

Ecology and Distribution of Lycopodiaceae Mirbel in Malaysia
Blumea 54, 2009: 269–271 www.ingentaconnect.com/content/nhn/blumea RESEARCH ARTICLE doi:10.3767/000651909X476265 Ecology and distribution of Lycopodiaceae Mirbel in Malaysia G. Rusea1, K. Claysius1, S. Runi1,2, U. Joanes2, K.M. Haja Maideen3, A. Latiff 3 Key words Abstract This paper is the first account to discuss the distribution, ecology and habitats of the Lycopodiaceae in Malaysia. Lycopodiaceae are widely distributed throughout Malaysia with respect to altitudes and environmental distribution conditions but most abundantly found in hill forest and lower montane forest, terrestrial as well as epiphytic, in ecology shaded or semi-shaded places with relatively high humidity. Pahang in Peninsular Malaysia and Sabah in Borneo habitats have the highest species diversity in terms of the number of species collected. Lycopodiaceae Published on 30 October 2009 INTRODUCTION RESULTS AND DISCUSSION The Lycopodiaceae s.l. are an ancient (Correll 1956) and In Malaysia, the family comprises 32 species including 11 va- probably monophyletic family without close living relatives rieties that are found in various altitudes and vegetation types, and have a virtually cosmopolitan distribution (Øllgaard 1992). sometimes in a restricted area (Table 1). The estimated number of species ranges from approximately Lycopodiella cernua has the widest distribution in Malaysia 300 to more than 400 around the world (Wikström 2001). It and is most common on acid soils and occurs along forest consists of three genera namely Huperzia, Lycopodium and fringes, along roadside, hillsides and mountain slopes fol- Lycopodiella. Worldwide the estimated number of species for lowed by Huperzia carinata and H. pinifolia, which occur on both Lycopodium and Lycopodiella is about 40 (Wikström & tree branches. -

Introduction to Botany. Lecture 35
Questions and answers Spermatophyta, seed plants Introduction to Botany. Lecture 35 Alexey Shipunov Minot State University November 28, 2011 Shipunov BIOL 154.35 Questions and answers Spermatophyta, seed plants Outline 1 Questions and answers 2 Spermatophyta, seed plants Classes of seed plants Conifers Gnetophytes Shipunov BIOL 154.35 Questions and answers Spermatophyta, seed plants Outline 1 Questions and answers 2 Spermatophyta, seed plants Classes of seed plants Conifers Gnetophytes Shipunov BIOL 154.35 Leaves-emergencies Leaves of lycopods Questions and answers Spermatophyta, seed plants Previous final question: the answer What are microphylls? Shipunov BIOL 154.35 Questions and answers Spermatophyta, seed plants Previous final question: the answer What are microphylls? Leaves-emergencies Leaves of lycopods Shipunov BIOL 154.35 Questions and answers Spermatophyta, seed plants Pteridophyta classes 1 2 3 4 5 6 7 8 9 10 Lycopodiopsida 1 0 0 1 0 0 1 1 0 0 Equisetopsida 0 1 0 1 0 1 0 1 0 1 Psilotopsida 0 1 1 0 0 0 0 0 0 1 Ophioglossopsida 0 1 0 0 0 0 1 0 0 0 Marattiopsida 0 1 1 0 0 1 0 1 1 0 Pteridopsida 1 1 0 0 1 1 0 1 1 0 1 Big (> 1,000 species); 2 Megaphyllous; 3 Synangia; 4 Strobilus; 5 Leptosporangia; 6 Terrestrial gametophyte; 7 Biflagellate sperm; 8 Roots; 9 Fronds; 10 Reduced leaves (enatia and scales). Characters are not necessary relevant to all members of class. Shipunov BIOL 154.35 Classes of seed plants Questions and answers Conifers Spermatophyta, seed plants Gnetophytes Spermatophyta, seed plants Classes of seed plants Shipunov BIOL 154.35 Classes of seed plants Questions and answers Conifers Spermatophyta, seed plants Gnetophytes Spermatophyta: seed plants ≈ 600 species of non-angiosperms and ≈ 250; 000 species of angiosperms Sporic life cycle with sporophyte predominance and seed Gametophyte is reduced to cells inside ovule or inside pollen grain.