263 Determining the Amount of Nucleic Acids in Medicinal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RI Equisetopsida and Lycopodiopsida.Indd
IIntroductionntroduction byby FFrancisrancis UnderwoodUnderwood Rhode Island Equisetopsida, Lycopodiopsida and Isoetopsida Special Th anks to the following for giving permission for the use their images. Robbin Moran New York Botanical Garden George Yatskievych and Ann Larson Missouri Botanical Garden Jan De Laet, plantsystematics.org Th is pdf is a companion publication to Rhode Island Equisetopsida, Lycopodiopsida & Isoetopsida at among-ri-wildfl owers.org Th e Elfi n Press 2016 Introduction Formerly known as fern allies, Horsetails, Club-mosses, Fir-mosses, Spike-mosses and Quillworts are plants that have an alternate generation life-cycle similar to ferns, having both sporophyte and gametophyte stages. Equisetopsida Horsetails date from the Devonian period (416 to 359 million years ago) in earth’s history where they were trees up to 110 feet in height and helped to form the coal deposits of the Carboniferous period. Only one genus has survived to modern times (Equisetum). Horsetails Horsetails (Equisetum) have jointed stems with whorls of thin narrow leaves. In the sporophyte stage, they have a sterile and fertile form. Th ey produce only one type of spore. While the gametophytes produced from the spores appear to be plentiful, the successful reproduction of the sporophyte form is low with most Horsetails reproducing vegetatively. Lycopodiopsida Lycopodiopsida includes the clubmosses (Dendrolycopodium, Diphasiastrum, Lycopodiella, Lycopodium , Spinulum) and Fir-mosses (Huperzia) Clubmosses Clubmosses are evergreen plants that produce only microspores that develop into a gametophyte capable of producing both sperm and egg cells. Club-mosses can produce the spores either in leaf axils or at the top of their stems. Th e spore capsules form in a cone-like structures (strobili) at the top of the plants. -

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

Effects of Lycopodium Clavatum and Equisetum Arvense Extracts from Western Romania
Romanian Biotechnological Letters Vol. , No. x, Copyright © 2016 University of Bucharest Printed in Romania. All rights reserved ORIGINAL PAPER Effects of Lycopodium clavatum and equisetum arvense extracts from western Romania Received for publication, July, 07, 2014 Accepted, October, 13, 2015 MARIA SUCIU1, FELIX AUREL MIC1, LUCIAN BARBU-TUDORAN2, VASILE MUNTEAN2, ALEXANDRA TEODORA GRUIA3,* 1University of Medicine and Pharmacy “Victor Babes”, Department of Functional Sciences, Timisoara, 2, Eftimie Murgu Sq., Timisoara, 300041, Timis County, Romania 2Babes-Bolyai University, Biology and Geology Department, 5-7 Clinicilor Str., Cluj-Napoca, 400084, Cluj County, Romania. 3Emergency Clinical County Hospital Timisoara, Regional Centre for Transplant Immunology Department, 10, Iosif Bulbuca Blvd., Timisoara, 300736, Timis County, Romania. *Address for correspondence to: [email protected], 10, Iosif Bulbuca Blvd., Timisoara, 300736, Timis County, Romania. Abbreviations: ALT–alanin transaminases, AST–aspartate transaminases, GC-MS–gas chromatograph coupled with mass spectrometry. Abstract Plants have always excited interest because of their active principles that could be a source of healing in various affections. The aim of this study was to demonstrate that the hepatoprotective and antimicrobial effects of Lycopodium clavatum and Equisetum arvense from the Western parts of Romania (Arad County) are not as pronounced as described in literature, against xenobiotic intoxication or microbial infection. To identify the plants active compounds, -
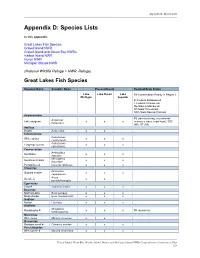
Species Lists
Appendix D: Species Lists Appendix D: Species Lists In this appendix: Great Lakes Fish Species Gravel Island NWR Gravel Island and Green Bay NWRs Harbor Island NWR Huron NWR Michigan Islands NWR (National Wildlife Refuge = NWR, Refuge) Great Lakes Fish Species Common Name Scientific Name Present/Absent Regional/State Status Lake Lake Huron Lake R3-Conservation Priority in Region 3 Michigan Superior E- Federal Endangered T-Federal Threatened SE-State Endangered ST-State Threatened SSC-State Special Concern Acipenseridae R3 (rare/declining, recreational/ Acipenser Lake sturgeon x x x economic value, tribal trust), SSC fulvescens (WI), ST (MI) Amiidae Bowfin Amia calva x x Catostomidae Catostomus White sucker x x x commersoni Catostomus Longnose sucker x x x catostomus Centrarchidae Ambloplites Rockbass x x x rupestris Micropterus Smallmouth bass x x x dolomieui Pumpkinseed Lepomis gibbosus x x x Clupeidae Dorosoma Gizzard shad # x x x cepedianum Alosa Alewife # x x pseudoharengus Cyprinidae Carp # Cyprinus Carpio x x x Esocidae Northern pike Esox Lucieus x x x Muskellunge Esox masquinongy x x x Gadidae Burbot Lota lota x x x Gobiidae Neogobius Round goby # x x x R3 (nuisance) melanostomus Moronidae White bass Morone chrysops x x Osmeridae Rainbow smelt # Osmerus mordax x x x Percichthyidae White perch # Morone americana x x x Gravel Island, Green Bay, Harbor Island, Huron, and Michigan Islands NWRs/Comprehensive Conservation Plan 221 Appendix D: Species Lists Common Name Scientific Name Present/Absent Regional/State Status Percidae R3 (rare/declining, -

Ecology and Distribution of Lycopodiaceae Mirbel in Malaysia
Blumea 54, 2009: 269–271 www.ingentaconnect.com/content/nhn/blumea RESEARCH ARTICLE doi:10.3767/000651909X476265 Ecology and distribution of Lycopodiaceae Mirbel in Malaysia G. Rusea1, K. Claysius1, S. Runi1,2, U. Joanes2, K.M. Haja Maideen3, A. Latiff 3 Key words Abstract This paper is the first account to discuss the distribution, ecology and habitats of the Lycopodiaceae in Malaysia. Lycopodiaceae are widely distributed throughout Malaysia with respect to altitudes and environmental distribution conditions but most abundantly found in hill forest and lower montane forest, terrestrial as well as epiphytic, in ecology shaded or semi-shaded places with relatively high humidity. Pahang in Peninsular Malaysia and Sabah in Borneo habitats have the highest species diversity in terms of the number of species collected. Lycopodiaceae Published on 30 October 2009 INTRODUCTION RESULTS AND DISCUSSION The Lycopodiaceae s.l. are an ancient (Correll 1956) and In Malaysia, the family comprises 32 species including 11 va- probably monophyletic family without close living relatives rieties that are found in various altitudes and vegetation types, and have a virtually cosmopolitan distribution (Øllgaard 1992). sometimes in a restricted area (Table 1). The estimated number of species ranges from approximately Lycopodiella cernua has the widest distribution in Malaysia 300 to more than 400 around the world (Wikström 2001). It and is most common on acid soils and occurs along forest consists of three genera namely Huperzia, Lycopodium and fringes, along roadside, hillsides and mountain slopes fol- Lycopodiella. Worldwide the estimated number of species for lowed by Huperzia carinata and H. pinifolia, which occur on both Lycopodium and Lycopodiella is about 40 (Wikström & tree branches. -
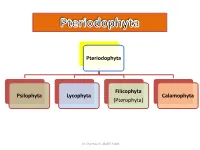
Pteriodophyta Psilophyta Lycophyta Filicophyta (Pterophyta) Calamophyta
Pteriodophyta Filicophyta Psilophyta Lycophyta Calamophyta (Pterophyta) Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora (Eligulatae) Order: Lycopodiales Family: Lycopodiaceae e.g. Lycopodium Subclass(2): Heterospora (Ligulatae) Order: Selaginellales Family: Selaginellaceae e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Phylum: Lepidophyta • It is characterized by: 1. The plant is differentiated into stem, leaves and roots. 2. Leaves are microphyllous (small, one vein and with no leaf gap). 3. The stele is protostele, siphonostele or polystele. 4. Protoxylem is exarch (ranging from a complete external cylinder to a polyarch stele in which there are many protoxylem ribs). 5. Sporangia are solitary, carried on special leaves (sporophylls). Sporophylls are usually collected in strobili. Dr. Shaimaa N. Abd El-Fatah . Among this phylum there are two independent evolutionary lines Heterospora (Ligulatae) Homospora (Eligulatae) • Characterized by presence • Eligulate (ligule lacking). of ligule (small outgrowth on • Homosporous (one type of the upper surface of the spores). leaf). • Heterosporous character (2 types of spores: microspores-- small, give rise to male gametophyte. & megaspores– larger, give rise to female gametophyte.) Dr. Shaimaa N. Abd El-Fatah • The phylum includes one class: Lycopodinae. • The class is classified into 4 orders: • Plants belong to the first order are homosporae (eligulatae), while the remaining three are heterosporae (ligulatae). Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora Subclass(2): Heterospora (Eligulatae) (Ligulatae) Order: Lycopodiales Order: Selaginellales Family: Lycopodiaceae Family: Selaginellaceae e.g. Lycopodium e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Lycopodiales This order is characterized by: 1. Homosporous and eligulatae. 2. Herbaceous without secondary growth. -

Conservation Assessment for Groundcedar and Stiff Clubmoss In
United States Department of Agriculture Conservation Assessment Forest Service for Groundcedar and Stiff Rocky Mountain Region Clubmoss in the Black Black Hills National Forest Hills National Forest South Custer, South Dakota Dakota and Wyoming March 2003 J.Hope Hornbeck, Deanna J. Reyher, Carolyn Sieg and Reed W. Crook Species Assessment of Groundcedar and Stiff Clubmoss in the Black Hills National Forest, South Dakota and Wyoming J. Hope Hornbeck, Deanna J. Reyher, Carolyn Hull Sieg and Reed W. Crook J. Hope Hornbeck is a Botanist with the Black Hills National Forest in Custer, South Dakota. She completed a B.S. in Environmental Biology at The University of Montana and a M.S. in Plant Biology at the University of Minnesota. Deanna J. Reyher is an Ecologist/Soil Scientist with the Black Hills National Forest in Custer, South Dakota. She completed a B.S. degree in Agronomy from the University of Nebraska. Carolyn Hull Sieg is a Research Plant Ecologist with the Rocky Mountain Research Station in Flagstaff, Arizona. She completed a B.S. in Wildlife Biology and M.S. in Range Science from Colorado State University and a Ph.D. in Range and Wildlife Management at Texas Tech University. Reed W. Crook is a Botanist with the Black Hills National Forest in Custer, South Dakota. He completed a B.S. in Botany at Brigham Young University, and a M.S. in Plant Morphology and Ph.D. in Plant Systematics at the University of Georgia. EXECUTIVE SUMMARY Stiff clubmoss (Lycopodium annotinum L.) and groundcedar (Lycopodium complanatum L.; synonym = Diphasiastrum complanatum [L.] Holub.) (Lycopodiaceae) are circumboreal clubmoss species that are widely distributed in North American boreal habitats. -

Lycopodiaceae Clubmoss Family
Lycopodiaceae Page | 46 clubmoss family Upwards of 15 genera comprise this ancient family. Perennial herbs, they somewhat resemble coarse mosses. The solitary sporangia are borne either in a terminal strobilus or are axillary with leaves. Spores are of equal size. In Nova Scotia we have four genera. A. Rhizomes absent; upright stems clustered; axillary sporangia; spores pitted. Huperzia aa. Rhizomes present; upright shoots alternate; sporangia aggregated into B terminal strobili, spores with netlike pattern. B. Strobili on leafy peduncles; mainly of wetland habitats. Lycopodiella bb. Strobili sessile or on peduncles with remote scant leaves; mainly of C dry upland places. C. Tips of stems 5–12mm in diameter; leaves in 6 ranks or Lycopodium more; leaves bristly, free for most of their length, not scalelike. cc. Distal shoots 2–6mm in diameter; leaves in 4–6 ranks, Diphasiastrum strongly overlapping (scalelike) and appressed along the stem with only tips free. Diphasiastrum Holub There are 15–20 species worldwide; numerous hybrids are possible. Generally these clubmosses are northern or subarctic in distribution. Nova Scotia has four species. Rhizomes bear sparse leaves that are reduced to scales, rooting from the lower surfaces. Upright stems are flattened or angled, with 2–5 branches. Leaves are arranged in four ranks and of two sizes. Sporophylls are smaller than unspecialized leaves. 1-7 Lycopodiaceae Key to species A. Plants < 12 cm tall; strobili sessile. Diphasiastrum sitchense Page | 47 aa. Stems 8–50cm; strobili on peduncles. B B. Branches square or angled, bluish. D. tristachyum bb. Branches flat; green. C C. Lateral branches irregular, annual winter bud constrictions D. -

81 Vascular Plant Diversity
f 80 CHAPTER 4 EVOLUTION AND DIVERSITY OF VASCULAR PLANTS UNIT II EVOLUTION AND DIVERSITY OF PLANTS 81 LYCOPODIOPHYTA Gleicheniales Polypodiales LYCOPODIOPSIDA Dipteridaceae (2/Il) Aspleniaceae (1—10/700+) Lycopodiaceae (5/300) Gleicheniaceae (6/125) Blechnaceae (9/200) ISOETOPSIDA Matoniaceae (2/4) Davalliaceae (4—5/65) Isoetaceae (1/200) Schizaeales Dennstaedtiaceae (11/170) Selaginellaceae (1/700) Anemiaceae (1/100+) Dryopteridaceae (40—45/1700) EUPHYLLOPHYTA Lygodiaceae (1/25) Lindsaeaceae (8/200) MONILOPHYTA Schizaeaceae (2/30) Lomariopsidaceae (4/70) EQifiSETOPSIDA Salviniales Oleandraceae (1/40) Equisetaceae (1/15) Marsileaceae (3/75) Onocleaceae (4/5) PSILOTOPSIDA Salviniaceae (2/16) Polypodiaceae (56/1200) Ophioglossaceae (4/55—80) Cyatheales Pteridaceae (50/950) Psilotaceae (2/17) Cibotiaceae (1/11) Saccolomataceae (1/12) MARATTIOPSIDA Culcitaceae (1/2) Tectariaceae (3—15/230) Marattiaceae (6/80) Cyatheaceae (4/600+) Thelypteridaceae (5—30/950) POLYPODIOPSIDA Dicksoniaceae (3/30) Woodsiaceae (15/700) Osmundales Loxomataceae (2/2) central vascular cylinder Osmundaceae (3/20) Metaxyaceae (1/2) SPERMATOPHYTA (See Chapter 5) Hymenophyllales Plagiogyriaceae (1/15) FIGURE 4.9 Anatomy of the root, an apomorphy of the vascular plants. A. Root whole mount. B. Root longitudinal-section. C. Whole Hymenophyllaceae (9/600) Thyrsopteridaceae (1/1) root cross-section. D. Close-up of central vascular cylinder, showing tissues. TABLE 4.1 Taxonomic groups of Tracheophyta, vascular plants (minus those of Spermatophyta, seed plants). Classes, orders, and family names after Smith et al. (2006). Higher groups (traditionally treated as phyla) after Cantino et al. (2007). Families in bold are described in found today in the Selaginellaceae of the lycophytes and all the pericycle or endodermis. Lateral roots penetrate the tis detail. -

Download Rory Hodd's CV
CURRICULUM VITAE RORY HODD B.Sc. (Hons.), Ph.D. EDUCATION 2007 – 2012: Botany and Plant Science, National University of Ireland, Galway, Ph.D. A study of the oceanic montane vegetation and bryophyte communities of Western Ireland and their potential response to climate change. 2003 – 2007: National University of Ireland, Galway, B.Sc. (Hons.) Botany (1st Class) Thesis: A study of the vegetation of the scree slopes of the Macgillycuddy’s Reeks, Co. Kerry. EMPLOYMENT HISTORY August 2010 – Present: Independent botanist and ecologist, Nimbosa Ecology Carrying out ecological survey work, with a strong conservation focus, specialising in highly detailed bryological, botanical and habitat survey, in a wide range of habitats throughout Ireland, as well as bryophyte identification and training in bryophyte and habitat identification. Clients include NPWS, Natural Resources Wales, Teagasc, Blackwater Regional Partnership, NUI Galway, BEC Consultants, Scott Cawley, Denyer Ecology, Mountaineering Ireland and Irish Rail. Currently undertaking monitoring of the EU Annex Habitat Calaminarian grassland across Ireland on behalf of NPWS. January 2017 – present: Visiting researcher/RIA Charlemont Scholar, Liverpool John Moores University, UK Initiating long-term experiments to monitor the impact of climate change on montane plant communities in North Wales. October 2015 – present: Associate Ecologist, BEC Consultants Lead Ecologist on project to monitor eight plant species listed in Annexes of the EU Habitats Directive. Acting as lead surveyor of populations -

Phenology and Function in Lycopod-Mucoromycotina Symbiosis
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.28.316760; this version posted September 29, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Phenology and function in lycopod-Mucoromycotina symbiosis. 2 3 Grace A. Hoysted1*, Martin I. Bidartondo2,3, Jeffrey G. Duckett4, Silvia Pressel4 and 4 Katie J. Field1 5 6 1Department of Animal and Plant Sciences, University of Sheffield, Sheffield, S10 7 2TN, UK 8 2Comparative Plant and Fungal Biology, Royal botanic Gardens, Kew, Richmond, 9 TW9 3DS, UK 10 3Department of Life Sciences, Imperial College London, London, SW7 2AZ, UK 11 4Department of Life Sciences, Natural History Museum, London, SW7 5BD, UK 12 13 *Corresponding author: 14 Grace A. Hoysted ([email protected]) 15 16 Words: 2338 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 bioRxiv preprint doi: https://doi.org/10.1101/2020.09.28.316760; this version posted September 29, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 35 Abstract 36 Lycopodiella inundata is a lycophyte with a complex life cycle. The gametophytes 37 and the juvenile, mature and retreating sporophytes form associations with 38 Mucoromycotina fine root endophyte (MFRE) fungi, being mycoheterotrophic as 39 gametophytes and mutualistic as mature sporophytes. However, the function of the 40 symbiosis across juvenile and retreating sporophyte life stages remains unknown. -

A Better Understanding of Pharmacological Activities and Uses
Journal of Pharmacognosy and Phytochemistry 2014; 3 (1): 207-210 ISSN 2278-4136 ISS N 2349-8234 A better understanding of pharmacological activities and JPP 2014; 3 (1): 207-210 Received: 10-04-2014 uses of phytochemicals of Lycopodium clavatum: A Accepted: 21-04-2014 review Jhilik Banerjee Post Graduate Department of Zoology, Jhilik Banerjee, Sunipa Biswas, Nithar Ranjan Madhu, Susanta Roy Karmakar, Midnapore College, Midnapore, West Surjyo Jyoti Biswas Bengal-721101 Sunipa Biswas ABSTRACT Post Graduate Department of Zoology, Lycopodium clavatum commonly known as club moss belongs to family Lycopodiaceae is a plant of great Midnapore College, Midnapore, West interest and is used in combating a wide variety of diseases in European and Asian countries. Crude Bengal-721101 extracts from various parts of the plant contain quinolizidine alkaloids. Several workers have reported various alkaloids derived from this plant and their therapeutic potentials, however, some reports are Nithar Ranjan Madhu Department of Zoology, Bajkul Milani available on toxicity studies of alkaloids of this plant. In such a scenario, there is a need for better Mahavidyalaya, Kismat Bajkul, West understanding of its ameliorating potential and its toxic actions. This review summarizes scientific Bengal, findings and suggests areas where further research is needed. Susanta Roy Karmakar Keywords: Lycopodium clavatum, alkaloids, pharmacology, antioxidant. Post Graduate Department of Zoology, Jhargram Raj College, Jhargram, 1. Introduction West Bengal Lycopodium clavatum, commonly known as Club moss, Clubfoot Moss, Foxtail, Ground Pine, Sulfer, Wolf’s Claw is one of the most widespread species belonging to family Lycopodiaceae. Surjyo Jyoti Biswas Post Graduate Department of Zoology, It is a pteridophyte which is abundantly found in tropical, subtropical and in many European Midnapore College, Midnapore, West countries.