Title: Impact of Trees and Forests on the Devonian Landscape and Weathering Processes with Implications to the Global Earth's System Properties – a Critical Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Contents Part III. the LAST FIFTY THOUSAND YEARS
STATE OF MICHIGAN Insects ................................................................... 60 DEPARTMENT OF CONSERVATION Worms And Others ................................................ 61 P. J. Hoffmaster, Director The Pleasant Peninsulas .............................................62 Man and his Towns ......................................................69 THEY NEED NOT VANISH A DISCUSSION OF THE NATURAL RESOURCES In Conclusion .................................................................71 OF MICHIGAN Part III. THE LAST FIFTY THOUSAND YEARS "THE GOOD EARTH" Edited by HELEN M. MARTIN ir, sunlight, water, and soil are essential for the Acontinuance of life—plant, animal, and human life, from contributions by on the earth. Of these four basic requirements, the soil Shirley W. Allen, Geo. C. S. Benson, University of is most directly subject to the care and management of Michigan man. However, the soil has frequently been the object of man's most careless use and abuse. It is, therefore, Stannard B. Bergquist, L. R. Schoenmann, H. C. most fitting that the eminent soil scientist, A. F. Beeskaw, J. H. Kraemer, W. F. Morofsky, J. A. Porter, E. Gustafson, should begin his book on soils and soil C. Sackrider, Michigan State College management with: G. S. Mclntire, H. M. Martin, O. F. Poindexter, C. F. "During his existence upon the earth, man has depended upon Welch, Department of Conservation the soil, either directly or indirectly, for the production of the materials used by him for food and clothing and, in part, for the M. G. Adams, Stream Control Commission production of those used for fuel and shelter as well. Grains, Frank DuMond, Public Museum, Grand Rapids fruits, and vegetables that serve him as food grow directly on the soil. Cotton and flax yield materials that are made into Lynn Heatley, High School, Midland. -

RI Equisetopsida and Lycopodiopsida.Indd
IIntroductionntroduction byby FFrancisrancis UnderwoodUnderwood Rhode Island Equisetopsida, Lycopodiopsida and Isoetopsida Special Th anks to the following for giving permission for the use their images. Robbin Moran New York Botanical Garden George Yatskievych and Ann Larson Missouri Botanical Garden Jan De Laet, plantsystematics.org Th is pdf is a companion publication to Rhode Island Equisetopsida, Lycopodiopsida & Isoetopsida at among-ri-wildfl owers.org Th e Elfi n Press 2016 Introduction Formerly known as fern allies, Horsetails, Club-mosses, Fir-mosses, Spike-mosses and Quillworts are plants that have an alternate generation life-cycle similar to ferns, having both sporophyte and gametophyte stages. Equisetopsida Horsetails date from the Devonian period (416 to 359 million years ago) in earth’s history where they were trees up to 110 feet in height and helped to form the coal deposits of the Carboniferous period. Only one genus has survived to modern times (Equisetum). Horsetails Horsetails (Equisetum) have jointed stems with whorls of thin narrow leaves. In the sporophyte stage, they have a sterile and fertile form. Th ey produce only one type of spore. While the gametophytes produced from the spores appear to be plentiful, the successful reproduction of the sporophyte form is low with most Horsetails reproducing vegetatively. Lycopodiopsida Lycopodiopsida includes the clubmosses (Dendrolycopodium, Diphasiastrum, Lycopodiella, Lycopodium , Spinulum) and Fir-mosses (Huperzia) Clubmosses Clubmosses are evergreen plants that produce only microspores that develop into a gametophyte capable of producing both sperm and egg cells. Club-mosses can produce the spores either in leaf axils or at the top of their stems. Th e spore capsules form in a cone-like structures (strobili) at the top of the plants. -

Introduction to Botany. Lecture 29
Kingdom Vegetabilia: plants Introduction to Botany. Lecture 29 Alexey Shipunov Minot State University November 12th, 2010 Shipunov BIOL 154.29 Kingdom Vegetabilia: plants Outline 1 Kingdom Vegetabilia: plants Bryophyta: mosses Pteridophyta: ferns and allies Shipunov BIOL 154.29 Bryophyta: mosses Kingdom Vegetabilia: plants Pteridophyta: ferns and allies Life cycle of mosses (picture from the board) Shipunov BIOL 154.29 Bryophyta: mosses Kingdom Vegetabilia: plants Pteridophyta: ferns and allies Three main groups (subphyla) Hepaticae—liverworts. Three classes, most primitive are Haplomitriopsida. Body has dorsal and ventral parts, sporogon bag-like, without columella, spores with elaters. Bryophytina—true mosses. Six classes, most important are Sphagnopsida (peat mosses), Polytrichopsida (haircap mosses) and Bryopsida. Body radial, sporogon long, with columella, spores without elaters. Anthocerotophytina—hornworts. One class. Body flattened, sporogon long, green, with columella and stomata, spores with elaters. Shipunov BIOL 154.29 Bryophyta: mosses Kingdom Vegetabilia: plants Pteridophyta: ferns and allies Haplomitrium gibbsiae, primitive liverwort Shipunov BIOL 154.29 Bryophyta: mosses Kingdom Vegetabilia: plants Pteridophyta: ferns and allies Elaters of liverworts (Lepidozia sp.) Shipunov BIOL 154.29 Bryophyta: mosses Kingdom Vegetabilia: plants Pteridophyta: ferns and allies Sphagnum sp. (Bryophyta, Sphagnopsida) with sporogons Shipunov BIOL 154.29 Bryophyta: mosses Kingdom Vegetabilia: plants Pteridophyta: ferns and allies Dawsonia -
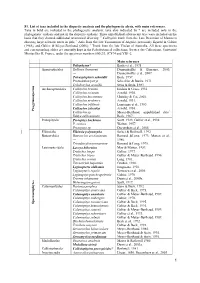
S1. List of Taxa Included in the Disparity Analysis and the Phylogenetic Alysis, with Main References
S1. List of taxa included in the disparity analysis and the phylogenetic alysis, with main references. Taxa in bold are included in the phylogenetic analysis; taxa also indicated by * are included only in the phylogenetic analysis and not in the disparity analysis. Three unpublished arborescent taxa were included on the basis that they showed additional anatomical diversity. 1 Callixylon trunk from the Late Devonian of Marrocco showing large sclerotic nests in pith; 2 Axis from the late Tournaisian of Algeria, previously figured in Galtier (1988), and Galtier & Meyer-Berthaud (2006); 3 Trunk from the late Viséan of Australia. All these specimens and corresponding slides are currently kept in the Paleobotanical collections, Service des Collections, Université Montpellier II, France, under the specimen numbers 600/2/3, JC874 and YB1-2. Main reference Psilophyton* Banks et al., 1975 Aneurophytales Rellimia thomsonii Dannenhoffer & Bonamo, 2003; --- Dannenhoffer et al., 2007. Tetraxylopteris schmidtii Beck, 1957. Proteokalon petryi Scheckler & Banks, 1971. Triloboxylon arnoldii Stein & Beck, 1983. s m Archaeopteridales Callixylon brownii Hoskin & Cross, 1951. r e Callixylon erianum Arnold, 1930. p s o Callixylon huronensis Chitaley & Cai, 2001. n Callixylon newberry Arnold, 1931. m y g Callixylon trifilievii Lemoigne et al., 1983. o r Callixylon zalesskyi Arnold, 1930. P Callixylon sp. Meyer-Berthaud, unpublished data1. Eddya sullivanensis Beck, 1967. Protopityales Protopitys buchiana Scott, 1923; Galtier et al., 1998. P. scotica Walton, 1957. Protopitys sp. Decombeix et al., 2005. Elkinsiales Elkinsia polymorpha Serbet & Rothwell, 1992. Buteoxylales Buteoxylon gordonianum Barnard &Long, 1973; Matten et al., --- 1980. Triradioxylon primaevum Barnard & Long, 1975. Lyginopteridales Laceya hibernica May & Matten, 1983. Tristichia longii Galtier, 1977. -

Earliest Record of Megaphylls and Leafy Structures, and Their Initial Diversification
Review Geology August 2013 Vol.58 No.23: 27842793 doi: 10.1007/s11434-013-5799-x Earliest record of megaphylls and leafy structures, and their initial diversification HAO ShouGang* & XUE JinZhuang Key Laboratory of Orogenic Belts and Crustal Evolution, School of Earth and Space Sciences, Peking University, Beijing 100871, China Received January 14, 2013; accepted February 26, 2013; published online April 10, 2013 Evolutionary changes in the structure of leaves have had far-reaching effects on the anatomy and physiology of vascular plants, resulting in morphological diversity and species expansion. People have long been interested in the question of the nature of the morphology of early leaves and how they were attained. At least five lineages of euphyllophytes can be recognized among the Early Devonian fossil plants (Pragian age, ca. 410 Ma ago) of South China. Their different leaf precursors or “branch-leaf com- plexes” are believed to foreshadow true megaphylls with different venation patterns and configurations, indicating that multiple origins of megaphylls had occurred by the Early Devonian, much earlier than has previously been recognized. In addition to megaphylls in euphyllophytes, the laminate leaf-like appendages (sporophylls or bracts) occurred independently in several dis- tantly related Early Devonian plant lineages, probably as a response to ecological factors such as high atmospheric CO2 concen- trations. This is a typical example of convergent evolution in early plants. Early Devonian, euphyllophyte, megaphyll, leaf-like appendage, branch-leaf complex Citation: Hao S G, Xue J Z. Earliest record of megaphylls and leafy structures, and their initial diversification. Chin Sci Bull, 2013, 58: 27842793, doi: 10.1007/s11434- 013-5799-x The origin and evolution of leaves in vascular plants was phology and evolutionary diversification of early leaves of one of the most important evolutionary events affecting the basal euphyllophytes remain enigmatic. -

Chians in Nova Scotia,. by J. \V. DAWSON, LL.D., F.R.S
440 Dawson-New Oarboniferous Batmchians of Nova Scotia. ART. XLVIII.-On a Recent Di~covery of Oarbo?Jifero1.l.s Batra chians in Nova Scotia,. by J. \V. DAWSON, LL.D., F.R.S. 1. General Remarks. THE erect Sigillarire enclosed in the f!andstone overlying coal-group 15 of Section XV, Division 4 of the SOIl.th Joggins section, are perhaps the most remarkable repositories ever dis covered of the remains of Paleozoic land animals. As I have shown in discussing their character in my memoirs on the South Joggins Coal FOl'mation,* and my "Acadian Geology," some of these trees became em bedded in sandy deposits, and being rendered hollow by decay of their inner bark and the crumbling of their woody axes, remained for a long time as open holes or pits, gradually filling with vegetable debris and the wash of rains and land floods. They thus became places of habitation for land snails and millepedes, and pit-falls into which the smaller batrachians, prowling for prey among the undergrowth of the coal forest, fell and were unable to extri cate themselves. In this way the successive layers of deposit became stored with skeletons of batrachians which they have retained in an admirable st.ate of preservation. Onlrone sandstone at the Joggins is known to contain these reptiliferous trees, though erect Sigillarire are known at. more than sixty different levels, and many of these erect stumps have been broken up in the hope of making such discoveries. In the past summer, however, shells of Pupa vetustu were found bv Mr. -

THE EVOLUTION of XYLEM ANATOMY in EARLY TRACHEOPHYTES by ELISABETH ANNE BERGMAN
Conquering the terrestrial environment: the evolution of xylem anatomy in early tracheophytes Item Type text; Electronic Thesis Authors Bergman, Elisabeth Anne Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 27/09/2021 03:01:29 Item License http://rightsstatements.org/vocab/InC/1.0/ Link to Item http://hdl.handle.net/10150/626731 CONQUERING THE TERRESTRIAL ENVIRONMENT: THE EVOLUTION OF XYLEM ANATOMY IN EARLY TRACHEOPHYTES By ELISABETH ANNE BERGMAN ____________________ A Thesis Submitted to The Honors College In Partial Fulfillment of the Bachelors Degree With Honors in Biology with an Emphasis in Biomedical Sciences THE UNIVERSITY OF ARIZONA D E C E M B E R 2 0 1 7 Approved by: ____________________________ Dr. Brian Enquist Department of Ecology and Evolutionary Biology Acknowledgements Many thanks go to all of those who made contributions, big and small, to my honors thesis, and more notably, my education. Foremost, I thank Dr. Brian Enquist for accepting me into his lab and serving as my mentor for two years. I appreciate all of the time he put in to meet with me and help me to develop my honors thesis. Additional thanks go to Dr. Sean Michaletz who first introduced me to the work that would eventually become my honors thesis. From the University of Santa Cruz, California, I thank Dr. -

Introduction to Botany. Lecture 35
Questions and answers Spermatophyta, seed plants Introduction to Botany. Lecture 35 Alexey Shipunov Minot State University November 28, 2011 Shipunov BIOL 154.35 Questions and answers Spermatophyta, seed plants Outline 1 Questions and answers 2 Spermatophyta, seed plants Classes of seed plants Conifers Gnetophytes Shipunov BIOL 154.35 Questions and answers Spermatophyta, seed plants Outline 1 Questions and answers 2 Spermatophyta, seed plants Classes of seed plants Conifers Gnetophytes Shipunov BIOL 154.35 Leaves-emergencies Leaves of lycopods Questions and answers Spermatophyta, seed plants Previous final question: the answer What are microphylls? Shipunov BIOL 154.35 Questions and answers Spermatophyta, seed plants Previous final question: the answer What are microphylls? Leaves-emergencies Leaves of lycopods Shipunov BIOL 154.35 Questions and answers Spermatophyta, seed plants Pteridophyta classes 1 2 3 4 5 6 7 8 9 10 Lycopodiopsida 1 0 0 1 0 0 1 1 0 0 Equisetopsida 0 1 0 1 0 1 0 1 0 1 Psilotopsida 0 1 1 0 0 0 0 0 0 1 Ophioglossopsida 0 1 0 0 0 0 1 0 0 0 Marattiopsida 0 1 1 0 0 1 0 1 1 0 Pteridopsida 1 1 0 0 1 1 0 1 1 0 1 Big (> 1,000 species); 2 Megaphyllous; 3 Synangia; 4 Strobilus; 5 Leptosporangia; 6 Terrestrial gametophyte; 7 Biflagellate sperm; 8 Roots; 9 Fronds; 10 Reduced leaves (enatia and scales). Characters are not necessary relevant to all members of class. Shipunov BIOL 154.35 Classes of seed plants Questions and answers Conifers Spermatophyta, seed plants Gnetophytes Spermatophyta, seed plants Classes of seed plants Shipunov BIOL 154.35 Classes of seed plants Questions and answers Conifers Spermatophyta, seed plants Gnetophytes Spermatophyta: seed plants ≈ 600 species of non-angiosperms and ≈ 250; 000 species of angiosperms Sporic life cycle with sporophyte predominance and seed Gametophyte is reduced to cells inside ovule or inside pollen grain. -

Giant Cladoxylopsid Trees Resolve Enigma of the Earth's Earliest Forest Stumps at Gilboa
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/6385893 Giant cladoxylopsid trees resolve enigma of the Earth's earliest forest stumps at Gilboa Article in Nature · May 2007 DOI: 10.1038/nature05705 · Source: PubMed CITATIONS READS 91 254 5 authors, including: Frank Mannolini Linda VanAller Hernick New York State Museum New York State Museum 8 PUBLICATIONS 160 CITATIONS 9 PUBLICATIONS 253 CITATIONS SEE PROFILE SEE PROFILE Ed Landing Christopher Berry New York State Museum Cardiff University 244 PUBLICATIONS 3,365 CITATIONS 48 PUBLICATIONS 862 CITATIONS SEE PROFILE SEE PROFILE All content following this page was uploaded by Ed Landing on 06 February 2017. The user has requested enhancement of the downloaded file. All in-text references underlined in blue are added to the original document and are linked to publications on ResearchGate, letting you access and read them immediately. Vol 446 | 19 April 2007 | doi:10.1038/nature05705 LETTERS Giant cladoxylopsid trees resolve the enigma of the Earth’s earliest forest stumps at Gilboa William E. Stein1, Frank Mannolini2, Linda VanAller Hernick2, Ed Landing2 & Christopher M. Berry3 The evolution of trees of modern size growing together in forests Middle Devonian (Eifelian) into the Carboniferous, were major fundamentally changed terrestrial ecosystems1–3. The oldest trees contributors to floras worldwide14. Traditionally considered inter- are often thought to be of latest Devonian age (about 380–360 Myr mediate between Lower Devonian vascular plants and ferns or old) as indicated by the widespread occurrence of Archaeopteris sphenopsids, we do not yet understand these plants well enough to (Progymnospermopsida)4. -

Curitiba, Southern Brazil
data Data Descriptor Herbarium of the Pontifical Catholic University of Paraná (HUCP), Curitiba, Southern Brazil Rodrigo A. Kersten 1,*, João A. M. Salesbram 2 and Luiz A. Acra 3 1 Pontifical Catholic University of Paraná, School of Life Sciences, Curitiba 80.215-901, Brazil 2 REFLORA Project, Curitiba, Brazil; [email protected] 3 Pontifical Catholic University of Paraná, School of Life Sciences, Curitiba 80.215-901, Brazil; [email protected] * Correspondence: [email protected]; Tel.: +55-41-3721-2392 Academic Editor: Martin M. Gossner Received: 22 November 2016; Accepted: 5 February 2017; Published: 10 February 2017 Abstract: The main objective of this paper is to present the herbarium of the Pontifical Catholic University of Parana’s and its collection. The history of the HUCP had its beginning in the middle of the 1970s with the foundation of the Biology Museum that gathered both botanical and zoological specimens. In April 1979 collections were separated and the HUCP was founded with preserved specimens of algae (green, red, and brown), fungi, and embryophytes. As of October 2016, the collection encompasses nearly 25,000 specimens from 4934 species, 1609 genera, and 297 families. Most of the specimens comes from the state of Paraná but there were also specimens from many Brazilian states and other countries, mainly from South America (Chile, Argentina, Uruguay, Paraguay, and Colombia) but also from other parts of the world (Cuba, USA, Spain, Germany, China, and Australia). Our collection includes 42 fungi, 258 gymnosperms, 299 bryophytes, 2809 pteridophytes, 3158 algae, 17,832 angiosperms, and only one type of Mimosa (Mimosa tucumensis Barneby ex Ribas, M. -

Belowground Rhizomes in Paleosols: the Hidden Half of an Early Devonian Vascular Plant
Belowground rhizomes in paleosols: The hidden half of an Early Devonian vascular plant Jinzhuang Xuea,b,1, Zhenzhen Denga, Pu Huanga, Kangjun Huanga, Michael J. Bentonc, Ying Cuid, Deming Wanga, Jianbo Liua, Bing Shena, James F. Basingere, and Shougang Haoa aThe Key Laboratory of Orogenic Belts and Crustal Evolution, School of Earth and Space Sciences, Peking University, Beijing 100871, People’s Republic of China; bKey Laboratory of Economic Stratigraphy and Palaeogeography, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing 210008, People’s Republic of China; cSchool of Earth Sciences, University of Bristol, Bristol BS8 1RJ, United Kingdom; dDepartment of Earth Sciences, Dartmouth College, Hanover, NH 03755; and eDepartment of Geological Sciences, University of Saskatchewan, Saskatoon, SK S7N 5E2, Canada Edited by Donald E. Canfield, Institute of Biology and Nordic Center for Earth Evolution, University of Southern Denmark, Odense M., Denmark, and approved June 28, 2016 (received for review March 28, 2016) The colonization of terrestrial environments by rooted vascular because of a poor fossil record, it remains quite unclear how the plants had far-reaching impacts on the Earth system. However, “hidden half” ecosystem (24), with buried structures growing in soils, the belowground structures of early vascular plants are rarely functioned during the early stage of vascular plant radiation. Such a documented, and thus the plant−soil interactions in early terres- knowledge gap hinders a deep understanding of the ecology of early trial ecosystems are poorly understood. Here we report the earli- plants and their roles in terrestrial environments. est rooted paleosols (fossil soils) in Asia from Early Devonian In this article, we report well-preserved plant traces from the deposits of Yunnan, China. -

PLANT SCIENCE Bulletin Summer 2013 Volume 59 Number 2
PLANT SCIENCE Bulletin Summer 2013 Volume 59 Number 2 1st Place Triarch Botanical Images Student Travel Awards Ricardo Kriebel The New York Botanical Garden Flower of Miconia arboricola (Melastomataceae: Miconieae) in late anthesis In This Issue.............. Dr. Thomas Ranker and The BSA awards many for their PLANTS Recipients excel in others elected to serve the contributions.....p. 36 botany ......p. 15 BSA.....p. 35 From the Editor PLANT SCIENCE Every year this is one of my favorite issues of BULLETIN Plant Science Bulletin because we get to recognize Editorial Committee the accomplishments of some of our most worthy Volume 59 members. The Merit Awardees have been elected to the most select group of professional botanists in North America. Begun at the Fiftieth Anniversary Elizabeth Schussler (2013) meeting, 55 years ago, the Merit Award recognizes Department of Ecology & individuals for their outstanding contributions to the Evolutionary Biology mission of the Botanical Society. These are people University of Tennessee whose names we recognize from their publications, Knoxville, TN 37996-1610 presentations, and service to the society. They are [email protected] leaders at their own institutions, in the Botanical Society and in other scientific organizations. What I find more interesting, though, are the younger members being recognized for their Christopher Martine potential. These are graduate students beginning (2014) to make their mark in botanical research and being Department of Biology invested with the opportunity to help direct the Bucknell University evolution of the Society. They are also undergraduates Lewisburg, PA 17837 being recognized by their mentors for their initiative, [email protected] enthusiasm and drive to make discoveries and share their love of plants with others.