Silurian to Triassic Plant and Hexapod Clades and Their Associations: New Data, a Review, and Interpretations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prepared in Cooperation with the Lllinois State Museum, Springfield
Prepared in cooperation with the lllinois State Museum, Springfield Richard 1. Leary' and Hermann W. Pfefferkorn2 ABSTRACT The Spencer Farm Flora is a compression-impression flora of early Pennsylvanian age (Namurian B, or possibly Namurian C) from Brown County, west-central Illinois. The plant fossils occur in argillaceous siltstones and sand- stones of the Caseyville Formation that were deposited in a ravine eroded in Mississippian carbonate rocks. The plant-bearing beds are the oldest deposits of Pennsylva- nian age yet discovered in Illinois. They were formed be- fore extensive Pennsylvanian coal swamps developed. The flora consists of 29 species and a few prob- lematical forms. It represents an unusual biofacies, in which the generally rare genera Megalopteris, Lesleya, Palaeopteridium, and Lacoea are quite common. Noegger- athiales, which are seldom present in roof-shale floras, make up over 20 percent of the specimens. The Spencer Farm Flora is an extrabasinal (= "upland1') flora that was grow- ing on the calcareous soils in the vicinity of the ravine in which they were deposited. It is suggested here that the Noeggerathiales may belong to the Progymnosperms and that Noeggerathialian cones might be derived from Archaeopteris-like fructifica- tions. The cone genus Lacoea is intermediate between Noeggerathiostrobus and Discini tes in its morphology. Two new species, Lesleya cheimarosa and Rhodeop- teridi urn phillipsii , are described, and Gulpenia limbur- gensis is reported from North America for the first time. INTRODUCTION The Spencer Farm Flora (table 1) differs from other Pennsylvanian floras of the Illinois Basin. Many genera and species in the Spencer Farm Flora either have not been found elsewhere in the basin or are very l~uratorof Geology, Illinois State Museum, Springfield. -

New Insects from the Earliest Permian of Carrizo Arroyo (New Mexico, USA) Bridging the Gap Between the Carboniferous and Permian Entomofaunas
Insect Systematics & Evolution 48 (2017) 493–511 brill.com/ise New insects from the earliest Permian of Carrizo Arroyo (New Mexico, USA) bridging the gap between the Carboniferous and Permian entomofaunas Jakub Prokopa,* and Jarmila Kukalová-Peckb aDepartment of Zoology, Faculty of Science, Charles University, Viničná 7, CZ-128 43 Praha 2, Czech Republic bEntomology, Canadian Museum of Nature, Ottawa, ON, Canada K1P 6P4 *Corresponding author, e-mail: [email protected] Version of Record, published online 7 April 2017; published in print 1 November 2017 Abstract New insects are described from the early Asselian of the Bursum Formation in Carrizo Arroyo, NM, USA. Carrizoneura carpenteri gen. et sp. nov. (Syntonopteridae) demonstrates traits in hindwing venation to Lithoneura and Syntonoptera, both known from the Moscovian of Illinois. Carrizoneura represents the latest unambiguous record of Syntonopteridae. Martynovia insignis represents the earliest evidence of Mar- tynoviidae. Carrizodiaphanoptera permiana gen. et sp. nov. extends range of Diaphanopteridae previously restricted to Gzhelian. The re-examination of the type speciesDiaphanoptera munieri reveals basally coa- lesced vein MA with stem of R and RP resulting in family diagnosis emendation. Arroyohymen splendens gen. et sp. nov. (Protohymenidae) displays features in venation similar to taxa known from early and late Permian from the USA and Russia. A new palaeodictyopteran wing attributable to Carrizopteryx cf. arroyo (Calvertiellidae) provides data on fore wing venation previously unknown. Thus, all these new discoveries show close relationship between late Pennsylvanian and early Permian entomofaunas. Keywords Ephemeropterida; Diaphanopterodea; Megasecoptera; Palaeodictyoptera; gen. et sp. nov; early Asselian; wing venation Introduction The fossil record of insects from continental deposits near the Carboniferous-Permian boundary is important for correlating insect evolution with changes in climate and in plant ecosystems. -
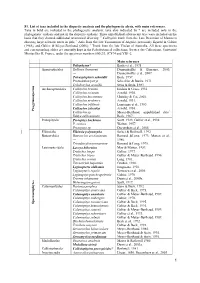
S1. List of Taxa Included in the Disparity Analysis and the Phylogenetic Alysis, with Main References
S1. List of taxa included in the disparity analysis and the phylogenetic alysis, with main references. Taxa in bold are included in the phylogenetic analysis; taxa also indicated by * are included only in the phylogenetic analysis and not in the disparity analysis. Three unpublished arborescent taxa were included on the basis that they showed additional anatomical diversity. 1 Callixylon trunk from the Late Devonian of Marrocco showing large sclerotic nests in pith; 2 Axis from the late Tournaisian of Algeria, previously figured in Galtier (1988), and Galtier & Meyer-Berthaud (2006); 3 Trunk from the late Viséan of Australia. All these specimens and corresponding slides are currently kept in the Paleobotanical collections, Service des Collections, Université Montpellier II, France, under the specimen numbers 600/2/3, JC874 and YB1-2. Main reference Psilophyton* Banks et al., 1975 Aneurophytales Rellimia thomsonii Dannenhoffer & Bonamo, 2003; --- Dannenhoffer et al., 2007. Tetraxylopteris schmidtii Beck, 1957. Proteokalon petryi Scheckler & Banks, 1971. Triloboxylon arnoldii Stein & Beck, 1983. s m Archaeopteridales Callixylon brownii Hoskin & Cross, 1951. r e Callixylon erianum Arnold, 1930. p s o Callixylon huronensis Chitaley & Cai, 2001. n Callixylon newberry Arnold, 1931. m y g Callixylon trifilievii Lemoigne et al., 1983. o r Callixylon zalesskyi Arnold, 1930. P Callixylon sp. Meyer-Berthaud, unpublished data1. Eddya sullivanensis Beck, 1967. Protopityales Protopitys buchiana Scott, 1923; Galtier et al., 1998. P. scotica Walton, 1957. Protopitys sp. Decombeix et al., 2005. Elkinsiales Elkinsia polymorpha Serbet & Rothwell, 1992. Buteoxylales Buteoxylon gordonianum Barnard &Long, 1973; Matten et al., --- 1980. Triradioxylon primaevum Barnard & Long, 1975. Lyginopteridales Laceya hibernica May & Matten, 1983. Tristichia longii Galtier, 1977. -

Is Ellipura Monophyletic? a Combined Analysis of Basal Hexapod
ARTICLE IN PRESS Organisms, Diversity & Evolution 4 (2004) 319–340 www.elsevier.de/ode Is Ellipura monophyletic? A combined analysis of basal hexapod relationships with emphasis on the origin of insects Gonzalo Giribeta,Ã, Gregory D.Edgecombe b, James M.Carpenter c, Cyrille A.D’Haese d, Ward C.Wheeler c aDepartment of Organismic and Evolutionary Biology, Museum of Comparative Zoology, Harvard University, 16 Divinity Avenue, Cambridge, MA 02138, USA bAustralian Museum, 6 College Street, Sydney, New South Wales 2010, Australia cDivision of Invertebrate Zoology, American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024, USA dFRE 2695 CNRS, De´partement Syste´matique et Evolution, Muse´um National d’Histoire Naturelle, 45 rue Buffon, F-75005 Paris, France Received 27 February 2004; accepted 18 May 2004 Abstract Hexapoda includes 33 commonly recognized orders, most of them insects.Ongoing controversy concerns the grouping of Protura and Collembola as a taxon Ellipura, the monophyly of Diplura, a single or multiple origins of entognathy, and the monophyly or paraphyly of the silverfish (Lepidotrichidae and Zygentoma s.s.) with respect to other dicondylous insects.Here we analyze relationships among basal hexapod orders via a cladistic analysis of sequence data for five molecular markers and 189 morphological characters in a simultaneous analysis framework using myriapod and crustacean outgroups.Using a sensitivity analysis approach and testing for stability, the most congruent parameters resolve Tricholepidion as sister group to the remaining Dicondylia, whereas most suboptimal parameter sets group Tricholepidion with Zygentoma.Stable hypotheses include the monophyly of Diplura, and a sister group relationship between Diplura and Protura, contradicting the Ellipura hypothesis.Hexapod monophyly is contradicted by an alliance between Collembola, Crustacea and Ectognatha (i.e., exclusive of Diplura and Protura) in molecular and combined analyses. -

The Associations Between Pteridophytes and Arthropods
FERN GAZ. 12(1) 1979 29 THE ASSOCIATIONS BETWEEN PTERIDOPHYTES AND ARTHROPODS URI GERSON The Hebrew University of Jerusalem, Faculty of Agriculture, Rehovot, Israel. ABSTRACT Insects belonging to 12 orders, as well as mites, millipedes, woodlice and tardigrades have been collected from Pterldophyta. Primitive and modern, as well as general and specialist arthropods feed on pteridophytes. Insects and mites may cause slight to severe damage, all plant parts being susceptible. Several arthropods are pests of commercial Pteridophyta, their control being difficult due to the plants' sensitivity to pesticides. Efforts are currently underway to employ insects for the biological control of bracken and water ferns. Although Pteridophyta are believed to be relatively resistant to arthropods, the evidence is inconclusive; pteridophyte phytoecdysones do not appear to inhibit insect feeders. Other secondary compounds of preridophytes, like prunasine, may have a more important role in protecting bracken from herbivores. Several chemicals capable of adversely affecting insects have been extracted from Pteridophyta. The litter of pteridophytes provides a humid habitat for various parasitic arthropods, like the sheep tick. Ants often abound on pteridophytes (especially in the tropics) and may help in protecting these plants while nesting therein. These and other associations are discussed . lt is tenatively suggested that there might be a difference in the spectrum of arthropods attacking ancient as compared to modern Pteridophyta. The Osmundales, which, in contrast to other ancient pteridophytes, contain large amounts of ·phytoecdysones, are more similar to modern Pteridophyta in regard to their arthropod associates. The need for further comparative studies is advocated, with special emphasis on the tropics. -

Conceptual Issues in Phylogeny, Taxonomy, and Nomenclature
Contributions to Zoology, 66 (1) 3-41 (1996) SPB Academic Publishing bv, Amsterdam Conceptual issues in phylogeny, taxonomy, and nomenclature Alexandr P. Rasnitsyn Paleontological Institute, Russian Academy ofSciences, Profsoyuznaya Street 123, J17647 Moscow, Russia Keywords: Phylogeny, taxonomy, phenetics, cladistics, phylistics, principles of nomenclature, type concept, paleoentomology, Xyelidae (Vespida) Abstract On compare les trois approches taxonomiques principales développées jusqu’à présent, à savoir la phénétique, la cladis- tique et la phylistique (= systématique évolutionnaire). Ce Phylogenetic hypotheses are designed and tested (usually in dernier terme s’applique à une approche qui essaie de manière implicit form) on the basis ofa set ofpresumptions, that is, of à les traits fondamentaux de la taxonomic statements explicite représenter describing a certain order of things in nature. These traditionnelle en de leur et particulier son usage preuves ayant statements are to be accepted as such, no matter whatever source en même temps dans la similitude et dans les relations de evidence for them exists, but only in the absence ofreasonably parenté des taxons en question. L’approche phylistique pré- sound evidence pleading against them. A set ofthe most current sente certains avantages dans la recherche de réponses aux phylogenetic presumptions is discussed, and a factual example problèmes fondamentaux de la taxonomie. ofa practical realization of the approach is presented. L’auteur considère la nomenclature A is made of the three -

Animal Phylum Poster Porifera
Phylum PORIFERA CNIDARIA PLATYHELMINTHES ANNELIDA MOLLUSCA ECHINODERMATA ARTHROPODA CHORDATA Hexactinellida -- glass (siliceous) Anthozoa -- corals and sea Turbellaria -- free-living or symbiotic Polychaetes -- segmented Gastopods -- snails and slugs Asteroidea -- starfish Trilobitomorpha -- tribolites (extinct) Urochordata -- tunicates Groups sponges anemones flatworms (Dugusia) bristleworms Bivalves -- clams, scallops, mussels Echinoidea -- sea urchins, sand Chelicerata Cephalochordata -- lancelets (organisms studied in detail in Demospongia -- spongin or Hydrazoa -- hydras, some corals Trematoda -- flukes (parasitic) Oligochaetes -- earthworms (Lumbricus) Cephalopods -- squid, octopus, dollars Arachnida -- spiders, scorpions Mixini -- hagfish siliceous sponges Xiphosura -- horseshoe crabs Bio1AL are underlined) Cubozoa -- box jellyfish, sea wasps Cestoda -- tapeworms (parasitic) Hirudinea -- leeches nautilus Holothuroidea -- sea cucumbers Petromyzontida -- lamprey Mandibulata Calcarea -- calcareous sponges Scyphozoa -- jellyfish, sea nettles Monogenea -- parasitic flatworms Polyplacophora -- chitons Ophiuroidea -- brittle stars Chondrichtyes -- sharks, skates Crustacea -- crustaceans (shrimp, crayfish Scleropongiae -- coralline or Crinoidea -- sea lily, feather stars Actinipterygia -- ray-finned fish tropical reef sponges Hexapoda -- insects (cockroach, fruit fly) Sarcopterygia -- lobed-finned fish Myriapoda Amphibia (frog, newt) Chilopoda -- centipedes Diplopoda -- millipedes Reptilia (snake, turtle) Aves (chicken, hummingbird) Mammalia -

THE ORIGIN and EVOLUTION of HYMENOPTEROUS INSECTS [P
THE ORIGIN AND EVOLUTION OF HYMENOPTEROUS INSECTS [p. 24] Chapter 3 EVOLUTION OF THE INFRACLASS SCARABAEONES A. P. Rasnitzyn* Dragonflies [order Odonata] and mayflies [order Ephemeroptera], which have retained a primitive thorax structure (absence of a cryptosternum) but are specialized in other respects, occupy the most isolated position in the infraclass. They are similar to each other in a number of characters, but the nature of the latter does not allow drawing a conclusion about any close relationship of the two orders. Indeed, the primitiveness of the structure of the thorax common to both of them, like any symplesiomorphy, is not evidence of a common origin. The adaptations of the larvae of mayflies and dragonflies to an aquatic way of life are different, and they show, rather, an independent transition to development in water. The loss of the ability to fold the wings as well is connected with different processes in them: in mayflies, with the ephemerality of the imago, the function of which is essentially limited to nuptial flight and oviposition; and in dragonflies, with the metamorphosis of the adult insect to an active aerial predator. It is highly probable that mayflies and dragonflies are independent evolutionary branches. The belonging of dragonflies and mayflies to a common trunk of Scarabaeones, that is, the isolation of them from Protoptera as part of a single trunk with the remaining members of the infraclass (except Protoptera), is confirmed by the metamorphosis of the style of the ninth segment in mayfly males into part of the copulatory organ [endophallus], and in dragonfly females into a sheath of the ovipositor. -

Ancient Noeggerathialean Reveals the Seed Plant Sister Group Diversified Alongside the Primary Seed Plant Radiation
Ancient noeggerathialean reveals the seed plant sister group diversified alongside the primary seed plant radiation Jun Wanga,b,c,1, Jason Hiltond,e, Hermann W. Pfefferkornf, Shijun Wangg, Yi Zhangh, Jiri Beki, Josef Pšenickaˇ j, Leyla J. Seyfullahk, and David Dilcherl,m,1 aState Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing 210008, China; bCenter for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, Nanjing 210008, China; cUniversity of Chinese Academy of Sciences, Shijingshan District, Beijing 100049, China; dSchool of Geography, Earth and Environmental Sciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, United Kingdom; eBirmingham Institute of Forest Research, University of Birmingham, Edgbaston, Birmingham B15 2TT, United Kingdom; fDepartment of Earth and Environmental Science, University of Pennsylvania, Philadelphia, PA 19104-6316; gState Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Xiangshan, Beijing 100093, China; hCollege of Paleontology, Shenyang Normal University, Key Laboratory for Evolution of Past Life in Northeast Asia, Ministry of Natural Resources, Shenyang 110034, China; iDepartment of Palaeobiology and Palaeoecology, Institute of Geology v.v.i., Academy of Sciences of the Czech Republic, 165 00 Praha 6, Czech Republic; jCentre of Palaeobiodiversity, West Bohemian Museum in Plzen, 301 36 Plzen, Czech Republic; kDepartment of Paleontology, Geozentrum, University of Vienna, 1090 Vienna, Austria; lIndiana Geological and Water Survey, Bloomington, IN 47404; and mDepartment of Geology and Atmospheric Science, Indiana University, Bloomington, IN 47405 Contributed by David Dilcher, September 10, 2020 (sent for review July 2, 2020; reviewed by Melanie Devore and Gregory J. -

Evolution of the Insects David Grimaldi and Michael S
Cambridge University Press 0521821495 - Evolution of the Insects David Grimaldi and Michael S. Engel Frontmatter More information EVOLUTION OF THE INSECTS Insects are the most diverse group of organisms to appear in the 3-billion-year history of life on Earth, and the most ecologically dominant animals on land. This book chronicles, for the first time, the complete evolutionary history of insects: their living diversity, relationships, and 400 million years of fossils. Whereas other volumes have focused on either living species or fossils, this is the first comprehensive synthesis of all aspects of insect evolution. Current estimates of phylogeny are used to interpret the 400-million-year fossil record of insects, their extinctions, and radiations. Introductory sections include the living species, diversity of insects, methods of reconstructing evolutionary relationships, basic insect structure, and the diverse modes of insect fossilization and major fossil deposits. Major sections cover the relationships and evolution of each order of hexapod. The book also chronicles major episodes in the evolutionary history of insects: their modest beginnings in the Devonian, the origin of wings hundreds of millions of years before pterosaurs and birds, the impact that mass extinctions and the explosive radiation of angiosperms had on insects, and how insects evolved the most complex societies in nature. Evolution of the Insects is beautifully illustrated with more than 900 photo- and electron micrographs, drawings, diagrams, and field photographs, many in full color and virtually all original. The book will appeal to anyone engaged with insect diversity: professional ento- mologists and students, insect and fossil collectors, and naturalists. David Grimaldi has traveled in 40 countries on 6 continents collecting and studying recent species of insects and conducting fossil excavations. -
Hanah, a Replacement Name for Hana Lau, Stokvis, Ofwegen & Reimer
A peer-reviewed open-access journal ZooKeys 918: 161–162Hanah (2020), a replacement name for Hana Lau, Stokvis, Ofwegen & Reimer, 2018 161 doi: 10.3897/zookeys.918.51123 CORRIGENDA http://zookeys.pensoft.net Launched to accelerate biodiversity research Corrigenda: Hanah, a replacement name for Hana Lau, Stokvis, Ofwegen & Reimer, 2018 (preoccupied name) Yee Wah Lau1, Frank R. Stokvis2, Leen van Ofwegen2, James D. Reimer1 1 University of the Ryukyus, Nishihara, Okinawa, Japan 2 Naturalis Biodiversity Center, Leiden, Netherlands Corresponding author: Yee Wah Lau ([email protected]) Academic editor: B. W. Hoeksema | Received 16 February 2020 | Accepted 19 February 2020 | Published 12 March 2020 http://zoobank.org/BA805D39-A503-4D6E-AAE1-4900823B6B18 Citation: Lau YW, Stokvis FR, van Ofwegen L, Reimer JD (2020) Corrigenda: Hanah, a replacement name for Hana Lau, Stokvis, Ofwegen & Reimer, 2018 (preoccupied name). ZooKeys 918: 161–162. https://doi.org/10.3897/ zookeys.918.51123 Abstract This is an addendum to the availability of the generic name Hana Lau, Stokvis, Ofwegen & Reimer, 2018 within the octocoral family Arulidae. Here, the replacement name Hanah is proposed, as Hana Lau, Stokvis, Ofwegen & Reimer, 2018 is a junior homonym to senior homonym Hana Kukalova-Peck, 1975, a genus within the family Hanidae of the Palaeozoic insect order Megasecoptera. Keywords Arulidae, Hana, homonymy, Octocorallia, replacement name, Stolonifera Class Anthozoa Subclass Octocorallia Order Alcyonacea Suborder Stolonifera Family Arulidae Genus Hanah nom. nov. Hana Lau, Stokvis, Ofwegen & Reimer, 2018. ZooKeys 790: 1–19. (Anthozoa: Oc- tocorallia: Alcyonacea: Stolonifera: Arulidae). Preoccupied by Hana Kukalova-Peck, 1975. Psyche 82: 1–19. (Insecta: Pterygota: Palaeoptera: Palaeodictyopteroidea: Megasecoptera: Hanidae). -

Mitochondrial Phylogenomics of Tenthredinidae (Hymenoptera: Tenthredinoidea) Supports the Monophyly of Megabelesesinae As a Subfamily
insects Article Mitochondrial Phylogenomics of Tenthredinidae (Hymenoptera: Tenthredinoidea) Supports the Monophyly of Megabelesesinae as a Subfamily Gengyun Niu 1,†, Sijia Jiang 2,†, Özgül Do˘gan 3 , Ertan Mahir Korkmaz 3 , Mahir Budak 3 , Duo Wu 1 and Meicai Wei 1,* 1 College of Life Sciences, Jiangxi Normal University, Nanchang 330022, China; [email protected] (G.N.); [email protected] (D.W.) 2 College of Forestry, Beijing Forestry University, Beijing 100083, China; [email protected] 3 Department of Molecular Biology and Genetics, Faculty of Science, Sivas Cumhuriyet University, Sivas 58140, Turkey; [email protected] (Ö.D.); [email protected] (M.B.); [email protected] (E.M.K.) * Correspondence: [email protected] † These authors contributed equally to this work. Simple Summary: Tenthredinidae is the most speciose family of the paraphyletic ancestral grade Symphyta, including mainly phytophagous lineages. The subfamilial classification of this family has long been problematic with respect to their monophyly and/or phylogenetic placements. This article reports four complete sawfly mitogenomes of Cladiucha punctata, C. magnoliae, Megabeleses magnoliae, and M. liriodendrovorax for the first time. To investigate the mitogenome characteristics of Tenthredinidae, we also compare them with the previously reported tenthredinid mitogenomes. To Citation: Niu, G.; Jiang, S.; Do˘gan, Ö.; explore the phylogenetic placements of these four species within this ecologically and economically Korkmaz, E.M.; Budak, M.; Wu, D.; Wei, important