Is This an Outbreak?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Primary Amoebic Meningoencephalitis Amoebic Meningoencephalitis Is Primary Ś
PØEHLEDOVÉ PRÁCE PØEHLEDOVÉ JE NEGLERIÓZA VEREJNO-ZDRAVOTNÍCKYM PROBLÉMOM? IS PRIMARY AMOEBIC MENINGOENCEPHALITIS (NAEGLERIASIS) A PUBLIC HEALTH PROBLEM? KATARÍNA TRNKOVÁ, LUCIA MAĎAROVÁ, CYRIL KLEMENT Regionálny úrad verejného zdravotníctva so sídlom v Banskej Bystrici, odbor lekárskej mikrobiológie SOUHRN Neglerióza alebo primárna amébová meningoencefalitída (PAM) je zriedkavé ochorenie CNS, pôvodcom ktorého je vo¾ne žijúca meòavka Naegleria fowleri. Medzi stovkami vo¾ne žijúcich meòaviek sú známe i ïalšie rody, ktorých zástupcovia sú schopní infikovaś èloveka a vyvolaś u neho ochorenie. Za patogény sú považovaní zástupcovia rodov Acanthamoeba a Naegleria a druhy Balamuthia mandrillaris a Sappi- nia diploidea. Infekcie spôsobené týmito organizmami vyvolávajú u ¾udí syndrómy v rozsahu od akútnych fatálnych ochorení po chronické, tkanivá napadajúce infekcie s granulomatóznymi prejavmi. Epidemiológia, imunológia, patológia a klinické prejavy týchto infekcií sa vzájomne ve¾mi líšia. Príspevok podáva preh¾ad o pôvodcovi ochorenia PAM, o jeho morfológii, životnom cykle, ekológii ako aj o patogenéze, symptomatike a spôsoboch laboratórnej diagnostiky negleriózy. K¾úèové slová: neglerióza, primárna amébová meningoencefalitída, epidemiológia, laboratórna diagnostika Naegleria fowleri SUMMARY Naegleriasis or primary amoebic meningoencephalitis (PAM) is invariably an acute, often fulminant infection of CNS caused by Naegleria fowleri, a small, free-living amoeba. Pathogenic free-living amoebae can cause serious illnesses in humans. The amoe- HYGIENA bae belonging to the genus Naegleria, Acanthamoeba and Balamuthia mandrillaris and Sappinia diploidea produce syndromes in man ranging from acute fatal disease to chronic tissue invasion with granulomatous manifestation. The purpose of this report is to describe the clinical history, treatment, pathology and methods of laboratory diagnostic of naegleriasis. Key words: primary amoebic meningoencephalitis, naegleriasis, epidemiology, laboratory diagnostics of Naegleria fowleri ÈÍSLO 2 Úvod Obr. -

SNF Mobility Model: ICD-10 HCC Crosswalk, V. 3.0.1
The mapping below corresponds to NQF #2634 and NQF #2636. HCC # ICD-10 Code ICD-10 Code Category This is a filter ceThis is a filter cellThis is a filter cell 3 A0101 Typhoid meningitis 3 A0221 Salmonella meningitis 3 A066 Amebic brain abscess 3 A170 Tuberculous meningitis 3 A171 Meningeal tuberculoma 3 A1781 Tuberculoma of brain and spinal cord 3 A1782 Tuberculous meningoencephalitis 3 A1783 Tuberculous neuritis 3 A1789 Other tuberculosis of nervous system 3 A179 Tuberculosis of nervous system, unspecified 3 A203 Plague meningitis 3 A2781 Aseptic meningitis in leptospirosis 3 A3211 Listerial meningitis 3 A3212 Listerial meningoencephalitis 3 A34 Obstetrical tetanus 3 A35 Other tetanus 3 A390 Meningococcal meningitis 3 A3981 Meningococcal encephalitis 3 A4281 Actinomycotic meningitis 3 A4282 Actinomycotic encephalitis 3 A5040 Late congenital neurosyphilis, unspecified 3 A5041 Late congenital syphilitic meningitis 3 A5042 Late congenital syphilitic encephalitis 3 A5043 Late congenital syphilitic polyneuropathy 3 A5044 Late congenital syphilitic optic nerve atrophy 3 A5045 Juvenile general paresis 3 A5049 Other late congenital neurosyphilis 3 A5141 Secondary syphilitic meningitis 3 A5210 Symptomatic neurosyphilis, unspecified 3 A5211 Tabes dorsalis 3 A5212 Other cerebrospinal syphilis 3 A5213 Late syphilitic meningitis 3 A5214 Late syphilitic encephalitis 3 A5215 Late syphilitic neuropathy 3 A5216 Charcot's arthropathy (tabetic) 3 A5217 General paresis 3 A5219 Other symptomatic neurosyphilis 3 A522 Asymptomatic neurosyphilis 3 A523 Neurosyphilis, -

Fidaxomicin Related Metabolic Acidosis
ABSTRACT NUMBER FIDAXOMICIN RELATED METABOLIC 5PSQ-121 ACIDOSIS: A CASE REPORT B. Serna Serrano1, A. Serrano Martínez 2, A. Valladolid Walsh 1, I. Pérez Alpuente 1, V. Lerma Gaude 1, M. Clemente Andújar 1, R. Aldaz Francés 1 Section 5: Patient Safety 1Complejo Hospitalario Universitario de Albacete, Pharmacy, Albacete, Spain. and Quality Assurance 2Complejo Hospitalario Universitario de Albacete, Hematology, Albacete, Spain. ATC code: A07 - Antidiarrheals, intestinal antiinflammatory/antiinfective agents BACKGROUND AND IMPORTANCE Fidaxomicin is a macrolide antibiotic used to treat intestinal Clostridium difficile (CD) infection in case of absence to metronidazole or vancomycin treatments. On the other hand, pharmacovigilance collects information, analyzes and notifies cases of suspected Adverse Drug Reactions (ADRs) in order to prevent them in the future. AIM AND To describe a case of metabolic acidosis OBJECTIVES in a patient treated with fidaxomicin and establish it is possible association. MATERIALS AND METHODS We describe the case of an 82-year-old male, diagnosed with multiple myeloma and treated with two full cycles of bortezomib-dexamethasone. He was referred to the Emergency department after presenting melenic diarrhea for one week. As a result, he was hospitalized and diagnosed with upper gastrointestinal bleeding, acute prerenal renal failure, mild thrombopenia, hypokalemia and hyponatremia. After fluid and electrolyte stabilization, it was decided to start with fidaxomicin 200 mg/12h due to fever, confusional syndrome, persistence of diarrhea and positive CD toxin test. The following constants were measured to confirm metabolic - acidosis: gas level of bicarbonate (HCO3 ), partial pressure of carbon dioxide (pCO2), hydrogen ion potential (pH) and anion GAP. The degree of drug/adverse reaction causality was evaluated using the Naranjo algorithm. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Issues of the Presence of Parasitic Protozoa in Surface Waters
E3S Web of Conferences 30, 01010 (2018) https://doi.org/10.1051/e3sconf/20183001010 Water, Wastewater and Energy in Smart Cities Issues of the presence of parasitic protozoa in surface waters Eliza Hawrylik1* 1 Department of Chemistry, Biology and Biotechnology, Faculty of Civil and Environmental Engineering, Bialystok University of Technology, Wiejska 45E Street, 15-351 Bialystok, Poland Abstract. Parasitic protozoa are very numerous organisms in the environment that play an important role in the spread of water-borne diseases. Water-borne epidemics caused by parasitic protozoa are noted throughout the world. Within these organisms, intestinal protozoa of the genera Cryptosporidium and Giardia are ones of the most serious health hazards for humans. This paper focuses on the problem of the presence of parasitic protozoa in surface waters. Characteristics of the most frequently recognized pathogens responsible for water-borne outbreaks were described, as well as sources of contamination and surface waters contamination due to protozoa of the genus Cryptosporidium and Giardia were presented. The methods of destroying the cysts and oocysts of parasitic protozoa used nowadays in the world were also presented in a review. 1 Occurrence of parasitic protozoa in surface water Parasitic protozoa are unicellular animal organisms that are very numerously isolated from natural waters, soils, food and media contaminated with animal manure and diseased humans. They are cosmopolitans, and are found in all countries of the world. They play an important role in the spread of water-borne diseases [1,2]. Water is the most common source of infection with protozoa, such as Entamoeba histolytica, Giardia intestinalis, Cryptosporidium sp., Cyclospora cayetanensis, Toxoplasma gondii. -

Toothless Aquatic Code Allows Deadly, Brain-Eating Zombie Amoeba to Flourish in Arizona Splash Pads and Water Playgrounds Sarah Pook*
IF WE ONLY HAD A BRAIN: Toothless Aquatic Code Allows Deadly, Brain-Eating Zombie Amoeba To Flourish in Arizona Splash Pads and Water Playgrounds Sarah Pook* I. INTRODUCTION It starts with a fever. A splitting headache. Vomiting, fatigue, an earache— then the secondary symptoms begin. Vision loss. Stiff neck. Lethargy, confusion, inability to walk, an aversion to light. Hallucinations. Doctors scramble to make a diagnosis, attempting treatment for bacterial meningitis, viral encephalitis, herpes, or other rare diseases, but nothing works.1 Finally, a coma. Death follows within three days.2 Diagnosis is usually done post-mortem: the culprit is primary amebic meningoencephalitis, or PAM.3 The disease is identified via a cerebrospinal fluid tap under a microscope. Peering in, you can see free living amoeba swimming around in the spinal fluid.4 * J.D. Candidate, 2020, Sandra Day O’Connor College of Law, Arizona State University; Editor-in-Chief, Arizona State Law Journal. I would like to thank Professor Tamara Herrera at Arizona State University for her insight and never-ending support as my faculty advisor, and Professor Marsha Howard at the University of Tulsa for her introduction to this fascinating topic. I would also like to thank my family for their love and encouragement. Finally, my heartfelt thanks to the wonderful Arizona State Law Journal staff and editors who bring our issues to publication—what a joy it is to work with all of you! 1. See Rikesh Baral & Binit Vaidya, Fatal Case of Amoebic Encephalitis Masquerading as Herpes, 5 OXFORD MED. CASE REPORTS. 146, 148 (2018), https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5934662/pdf/omy010.pdf [https://perma.cc/HSM6-WZZV]. -

Drug Consumption in Current Year (Period 201901
Page 1 Drug consumption in current year (Period 202001 - 202012) Wholesale ATC code Subgroup or chemical substance DDD/1000 inhab./day Hospital % Change % price/1000 € Hospital % Change % A ALIMENTARY TRACT AND METABOLISM 323,80 3 4 321 589 7 4 A01 STOMATOLOGICAL PREPARATIONS 14,28 4 12 2 090 9 8 A01A STOMATOLOGICAL PREPARATIONS 14,28 4 12 2 090 9 8 A01AA Caries prophylactic agents 11,90 3 14 663 8 9 A01AA01 sodium fluoride 11,90 3 14 610 8 10 A01AA03 olaflur - - - 53 1 -2 A01AB Antiinfectives for local oral treatment 2,36 8 2 1 266 10 15 A01AB03 chlorhexidine 2,02 6 -3 930 6 5 A01AB11 various 0,33 21 57 335 21 55 A01AB22 doxycycline - - - 0 - -100 A01AC Corticosteroids for local oral treatment - - - 113 1 -26 A01AC01 triamcinolone - - - 113 1 -26 A01AD Other agents for local oral treatment 0,02 0 -28 49 0 -32 A01AD02 benzydamine 0,02 0 -28 49 0 -32 A02 DRUGS FOR ACID RELATED DISORDERS 73,05 3 3 30 885 4 -5 A02A ANTACIDS 2,23 1 1 3 681 1 3 A02AA Magnesium compounds 0,07 22 -7 141 22 -7 A02AA04 magnesium hydroxide 0,07 22 -7 141 22 -7 A02AD Combinations and complexes of aluminium, 2,17 0 1 3 539 0 4 calcium and magnesium compounds A02AD01 ordinary salt combinations 2,17 0 1 3 539 0 4 A02B DRUGS FOR PEPTIC ULCER AND 70,82 3 3 27 205 5 -7 GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 0,17 7 -77 551 10 -29 A02BA02 ranitidine 0,00 1 -100 1 1 -100 A02BA03 famotidine 0,16 7 48 550 10 43 A02BB Prostaglandins 0,04 62 55 80 62 55 A02BB01 misoprostol 0,04 62 55 80 62 55 A02BC Proton pump inhibitors 69,26 3 4 23 531 4 -8 -

Taxonomy of Clinically Relevant Microorganisms*
OpenStax-CNX module: m58949 1 Taxonomy of Clinically Relevant Microorganisms* OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 1 Bacterial Pathogens The following tables list the species, and some higher groups, of pathogenic Eubacteria mentioned in the text. The classication of Bacteria, one of the three domains of life, is in constant ux as relationships become clearer through sampling of genetic sequences. Many groups at all taxonomic levels still have an undetermined relationship with other members of the phylogenetic tree of Bacteria. Bergey's Manual of Systematics of Archaea and Bacteria maintains a published list and descriptions of prokaryotic species. The tables here follow the taxonomic organization in the Bergey's Manual Taxonomic Outline.1 We have divided the species into tables corresponding to dierent bacterial phyla. The taxonomic rank of kingdom is not used in prokaryote taxonomy, so the phyla are the subgrouping below domain. Note that many bacterial phyla not represented by these tables. The species and genera are listed only under the class within each phylum. The names given to bacteria are regulated by the International Code of Nomenclature of Bacteria as maintained by the International Committee on Systematics or Prokaryotes. Phylum Actinobacteria Class Genus Species Related Diseases Corynebacterium diphtheriae Diphtheria Gardnerella vaginalis Bacterial vaginosis Micrococcus Opportunistic infections Actinobacteria Mycobacterium bovis Tuberculosis, primarily in cattle Mycobacterium leprae Hansen's disease continued on next page *Version 1.5: Apr 4, 2018 1:24 pm -0500 http://creativecommons.org/licenses/by/4.0/ 1Bergey's Manual Trust. Bergey's Manual of Systematics of Archaea and Bacteria, Taxonomic Outline. -

Drug Consumption at Wholesale Prices in 2017 - 2020
Page 1 Drug consumption at wholesale prices in 2017 - 2020 2020 2019 2018 2017 Wholesale Hospit. Wholesale Hospit. Wholesale Hospit. Wholesale Hospit. ATC code Subgroup or chemical substance price/1000 € % price/1000 € % price/1000 € % price/1000 € % A ALIMENTARY TRACT AND METABOLISM 321 590 7 309 580 7 300 278 7 295 060 8 A01 STOMATOLOGICAL PREPARATIONS 2 090 9 1 937 7 1 910 7 2 128 8 A01A STOMATOLOGICAL PREPARATIONS 2 090 9 1 937 7 1 910 7 2 128 8 A01AA Caries prophylactic agents 663 8 611 11 619 12 1 042 11 A01AA01 sodium fluoride 610 8 557 12 498 15 787 14 A01AA03 olaflur 53 1 54 1 50 1 48 1 A01AA51 sodium fluoride, combinations - - - - 71 1 206 1 A01AB Antiinfectives for local oral treatment 1 266 10 1 101 6 1 052 6 944 6 A01AB03 chlorhexidine 930 6 885 7 825 7 706 7 A01AB11 various 335 21 216 0 227 0 238 0 A01AB22 doxycycline - - 0 100 0 100 - - A01AC Corticosteroids for local oral treatment 113 1 153 1 135 1 143 1 A01AC01 triamcinolone 113 1 153 1 135 1 143 1 A01AD Other agents for local oral treatment 49 0 72 0 104 0 - - A01AD02 benzydamine 49 0 72 0 104 0 - - A02 DRUGS FOR ACID RELATED DISORDERS 30 885 4 32 677 4 35 102 5 37 644 7 A02A ANTACIDS 3 681 1 3 565 1 3 357 1 3 385 1 A02AA Magnesium compounds 141 22 151 22 172 22 155 19 A02AA04 magnesium hydroxide 141 22 151 22 172 22 155 19 A02AD Combinations and complexes of aluminium, 3 539 0 3 414 0 3 185 0 3 231 0 calcium and magnesium compounds A02AD01 ordinary salt combinations 3 539 0 3 414 0 3 185 0 3 231 0 A02B DRUGS FOR PEPTIC ULCER AND 27 205 5 29 112 4 31 746 5 34 258 8 -
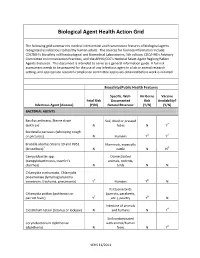
Biological Agent Health Action Grid
Biological Agent Health Action Grid The following grid summarizes medical intervention and transmission features of biological agents recognized as infectious to healthy human adults. The sources for foonote information include: CDC/NIH’s Biosafety in Microbiological and Biomedical Laboratories, 5th edition; CDC/HHS’s Advisory Committee on Immunization Practices; and the APHIS/CDC’s National Select Agent Registry/Select Agents Exclusion. This document is intended to serve as a general information guide. A full risk assessment needs to be prepared for the use of any infectious agent in a lab or animal research setting, and appropriate research compliance committee approvals obtained before work is initiated. Biosafety/Public Health Features Specific, Well- Air-Borne Vaccine Fetal Risk Documented Risk Availability? Infectious Agent (disease) (Y/N) Natural Reservoir (Y/N) (Y/N) BACTERIAL AGENTS Bacillus anthracis, Sterne strain Soil, dried or pressed (anthrax)1 N hides N Y2 Bordetella pertussis (whooping cough or pertussis) N Humans Y3 Y4 Brucella abortus Strains 19 and RB51 Mammals, especially (brucellosis)5 N cattle N N6 Campylobacter spp. Domesticated (campylobacteriosis, traveler's animals, rodents, diarrhea) N birds N N Chlamydia trachomatis, Chlamydia pneumoniae (lymphogranuloma venereum, trachoma, pneumonia) Y7 Humans Y8 N Psittacine birds Chlamydia psittaci (psittacosis or (parrots, parakeets, parrott fever) Y7 etc.), poultry Y8 N Intestine of animals Clostridium tetani (tetanus or lockjaw) N and humans N Y4 Soil contaminated Corynebacterium diphtheriae with animal/human (diphtheria) N feces N Y4 VEHS 11/2011 Specific, Well- Air-Borne Vaccine Fetal Risk Documented Risk Availability? Infectious Agent (disease) (Y/N) Natural Reservoir (Y/N) (Y/N) Francisella tularensis, subspecies Wild animals, esp. -

Estonian Statistics on Medicines 2013 1/44
Estonian Statistics on Medicines 2013 DDD/1000/ ATC code ATC group / INN (rout of admin.) Quantity sold Unit DDD Unit day A ALIMENTARY TRACT AND METABOLISM 146,8152 A01 STOMATOLOGICAL PREPARATIONS 0,0760 A01A STOMATOLOGICAL PREPARATIONS 0,0760 A01AB Antiinfectives and antiseptics for local oral treatment 0,0760 A01AB09 Miconazole(O) 7139,2 g 0,2 g 0,0760 A01AB12 Hexetidine(O) 1541120 ml A01AB81 Neomycin+Benzocaine(C) 23900 pieces A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+Thymol(dental) 2639 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+Cetylpyridinium chloride(gingival) 179340 g A01AD81 Lidocaine+Cetrimide(O) 23565 g A01AD82 Choline salicylate(O) 824240 pieces A01AD83 Lidocaine+Chamomille extract(O) 317140 g A01AD86 Lidocaine+Eugenol(gingival) 1128 g A02 DRUGS FOR ACID RELATED DISORDERS 35,6598 A02A ANTACIDS 0,9596 Combinations and complexes of aluminium, calcium and A02AD 0,9596 magnesium compounds A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 591680 pieces 10 pieces 0,1261 A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 1998558 ml 50 ml 0,0852 A02AD82 Aluminium aminoacetate+Magnesium oxide(O) 463540 pieces 10 pieces 0,0988 A02AD83 Calcium carbonate+Magnesium carbonate(O) 3049560 pieces 10 pieces 0,6497 A02AF Antacids with antiflatulents Aluminium hydroxide+Magnesium A02AF80 1000790 ml hydroxide+Simeticone(O) DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 34,7001 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 3,5364 A02BA02 Ranitidine(O) 494352,3 g 0,3 g 3,5106 A02BA02 Ranitidine(P) -

A Map of the Alternative Biomedical R&D Landscape
A map of the alternative biomedical R&D landscape ACKNOWLEDGEMENTS We would like to acknowledge all the engaged and active student leaders in UAEM who have supported, participated and activated around the critical global health issue of alternative biomedical R&D, the role of universities and ensuring access to medicines for all. Thanks as well as to the staff and board of UAEM. Special thanks to Els Torreele PhD, Director of the Open Society Public Health Program’s Access to Essential Medicines Initiative, for her advice, support and encouragement. And thanks to the team at signals.ca for providing us with their design talent and enthusiastically agreeing to complete this project in record time! AUTHOR BIOGRAPHIES Rachel Kiddell-Monroe LL.M is a humanitarian, a lawyer and an activist. She is Special Adviser to Universities Allied for Essential Medicines (UAEM) and served 6 years as President of the UAEM board of directors. She is also a member of the International Board of directors for Médecins Sans Frontières/Doctors Without Borders (MSF). She lives in Montreal and has worked in south east Asia with human rights and environmental grassroots activists as well as with MSF in Djibouti, Rwanda, the Democratic Republic of Congo and throughout Latin America. Rachel has also served as the Canadian director of MSF’s Access to Medicines Campaign. Rachel sits on the board of UAEM Europe, on the Advisory Council of UAEM Brazil and the board of the Young Professionals for Chronic Disease Network. Rachel is a Professor of Practice at McGill University, lectures on international development and is part of the Adult Clinical Ethics Committee at McGill University Health Centres.