The Selection and Use of Essential Medicines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MASCC/ESMO ANTIEMETIC GUIDELINE 2016 with Updates in 2019
1 ANTIEMETIC GUIDELINES: MASCC/ESMO MASCC/ESMO ANTIEMETIC GUIDELINE 2016 With Updates in 2019 Organizing and Overall Meeting Chairs: Matti Aapro, MD Richard J. Gralla, MD Jørn Herrstedt, MD, DMSci Alex Molassiotis, RN, PhD Fausto Roila, MD © Multinational Association of Supportive Care in CancerTM All rights reserved worldwide. 2 ANTIEMETIC GUIDELINES: MASCC/ESMO These slides are provided to all by the Multinational Association of Supportive Care in Cancer and can be used freely, provided no changes are made and the MASCC and ESMO logos, as well as date of the information are retained. For questions please contact: Matti Aapro at [email protected] Chair, MASCC Antiemetic Study Group or Alex Molassiotis at [email protected] Past Chair, MASCC Antiemetic Study Group 3 ANTIEMETIC GUIDELINES: MASCC/ESMO Consensus A few comments on this guideline set: • This set of guideline slides represents the latest edition of the guideline process. • This set of slides has been endorsed by the MASCC Antiemetic Guideline Committee and ESMO Guideline Committee. • The guidelines are based on the votes of the panel at the Copenhagen Consensus Conference on Antiemetic Therapy, June 2015. • Latest version: March 2016, with updates in 2019. 4 ANTIEMETIC GUIDELINES: MASCC/ESMO Changes: The Steering Committee has clarified some points: 2016: • A footnote clarified that aprepitant 165 mg is approved by regulatory authorities in some parts of the world ( although no randomised clinical trial has investigated this dose ). Thus use of aprepitant 80 mg in the delayed phase is only for those cases where aprepitant 125 mg is used on day 1. • A probable modification in pediatric guidelines based on the recent Cochrane meta-analysis is indicated. -

Pharmacokinetics of Salicylic Acid Following Intravenous and Oral Administration of Sodium Salicylate in Sheep
animals Article Pharmacokinetics of Salicylic Acid Following Intravenous and Oral Administration of Sodium Salicylate in Sheep Shashwati Mathurkar 1,*, Preet Singh 2 ID , Kavitha Kongara 2 and Paul Chambers 2 1 1B, He Awa Crescent, Waikanae 5036, New Zealand 2 School of Veterinary Sciences, College of Sciences, Massey University, Palmerston North 4474, New Zealand; [email protected] (P.S.); [email protected] (K.K.); [email protected] (P.C.) * Correspondence: [email protected]; Tel.: +64-221-678-035 Received: 13 June 2018; Accepted: 16 July 2018; Published: 18 July 2018 Simple Summary: Scarcity of non-steroidal anti-inflammatory drugs (NSAID) to minimise the pain in sheep instigated the current study. The aim of this study was to know the pharmacokinetic parameters of salicylic acid in New Zealand sheep after administration of multiple intravenous and oral doses of sodium salicylate (sodium salt of salicylic acid). Results of the study suggest that the half-life of the drug was shorter and clearance was faster after intravenous administration as compared to that of the oral administration. The minimum effective concentration required to produce analgesia in humans (16.8 µL) was achieved in sheep for about 0.17 h in the current study after intravenous administration of 100 and 200 mg/kg body weight of sodium salicylate. However, oral administration of these doses failed to achieve the minimum effective concentration as mentioned above. This study is of significance as it adds valuable information on pharmacokinetics and its variation due to breed, species, age, gender and environmental conditions. -

Expert Review 2
2021 Expert Committee on Selection and Use of Essential Medicines Application review I.1 Albendazole, mebendazole and praziquantel for the indication of treatment of (item number) taeniid cestode cysts Does the application adequately ☒ Yes address the issue of the public health ☐ need for the medicine? No ☐ Not applicable Comments: The larval stages of three taeniid cestode parasites, Echinococcus granulosus, Echinococcus multilocularis and Taenia solium, produce cysts in humans that are of medical relevance. The diseases caused by these parasitic cysts are called cystic echinococcosis (CE), alveolar echinococcosis (AE), and cysticercosis (being neurocysticercosis (NCC) the most common form) respectively, and they are recognised by WHO as neglected tropical diseases. NCC is mainly a disease of poverty that predominantly affects rural populations in Africa, Asia and Latin America. Access to diagnostic and treatment, to better manage epilepsy and other NCC is a challenge for the people affected in these communities due to the availability and costs of specialised diagnostic and care. Stigma and social discrimination also mean that many people try to “hide” the disease. Briefly summarize the role of the The only real options for treatment of CE are albendazole (ALB) and Mebendazole proposed medicine(s) relative to other (MEB). ALB is the drug of choice as it has better bioavailability. ALB is also preferred to therapeutic agents currently included in MEB, because MEB requires a higher dose and a higher pill burden, for example, an the Model List, or available in the adult patient would require 8 tablets/day of MEB compared with 2 tablets/day ALB. market. ALB and praziquantel ( PZQ) are the only drugs used for the antiparasitic treatment of NCC. -

Clinical Practice Guideline for Emergency Department Ketamine Dissociative Sedation: 2011 Update
PAIN MANAGEMENT/CONCEPTS Clinical Practice Guideline for Emergency Department Ketamine Dissociative Sedation: 2011 Update Steven M. Green, MD, Mark G. Roback, MD, Robert M. Kennedy, MD, Baruch Krauss, MD, EdM From the Department of Emergency Medicine, Loma Linda University Medical Center and Children’s Hospital, Loma Linda, CA (Green); the Department of Pediatrics, University of Minnesota, Minneapolis, MN (Roback); the Division of Emergency Medicine, St. Louis Children’s Hospital, Washington University, St. Louis, MO (Kennedy); and the Division of Emergency Medicine, Children’s Hospital Boston and Department of Pediatrics, Harvard Medical School, Boston, MA (Krauss). We update an evidence-based clinical practice guideline for the administration of the dissociative agent ketamine for emergency department procedural sedation and analgesia. Substantial new research warrants revision of the widely disseminated 2004 guideline, particularly with respect to contraindications, age recommendations, potential neurotoxicity, and the role of coadministered anticholinergics and benzodiazepines. We critically discuss indications, contraindications, personnel requirements, monitoring, dosing, coadministered medications, recovery issues, and future research questions for ketamine dissociative sedation. [Ann Emerg Med. 2011;xx:xxx.] 0196-0644/$-see front matter Copyright © 2011 by the American College of Emergency Physicians. doi:10.1016/j.annemergmed.2010.11.030 INTRODUCTION thalamocortical and limbic systems, effectively dissociating the The dissociative -
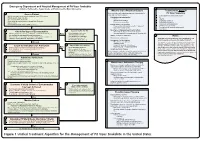
Snake Bite Protocol
Lavonas et al. BMC Emergency Medicine 2011, 11:2 Page 4 of 15 http://www.biomedcentral.com/1471-227X/11/2 and other Rocky Mountain Poison and Drug Center treatment of patients bitten by coral snakes (family Ela- staff. The antivenom manufacturer provided funding pidae), nor by snakes that are not indigenous to the US. support. Sponsor representatives were not present dur- At the time this algorithm was developed, the only ing the webinar or panel discussions. Sponsor represen- antivenom commercially available for the treatment of tatives reviewed the final manuscript before publication pit viper envenomation in the US is Crotalidae Polyva- ® for the sole purpose of identifying proprietary informa- lent Immune Fab (ovine) (CroFab , Protherics, Nash- tion. No modifications of the manuscript were requested ville, TN). All treatment recommendations and dosing by the manufacturer. apply to this antivenom. This algorithm does not con- sider treatment with whole IgG antivenom (Antivenin Results (Crotalidae) Polyvalent, equine origin (Wyeth-Ayerst, Final unified treatment algorithm Marietta, Pennsylvania, USA)), because production of The unified treatment algorithm is shown in Figure 1. that antivenom has been discontinued and all extant The final version was endorsed unanimously. Specific lots have expired. This antivenom also does not consider considerations endorsed by the panelists are as follows: treatment with other antivenom products under devel- opment. Because the panel members are all hospital- Role of the unified treatment algorithm -

ALBENDAZOLE (Extrapolation to All Ruminants)
European Medicines Agency Veterinary Medicines and Inspections EMEA/MRL/865/03-FINAL June 2004 COMMITTEE FOR MEDICINAL PRODUCTS FOR VETERINARY USE ALBENDAZOLE (Extrapolation to all ruminants) SUMMARY REPORT (3) 1. Albendazole is a benzimidazole carbamate, used for the treatment of gastrointestinal infestations with roundworms, lungworms and tapeworms and adult flukes of Fasciola hepatica. Albendazole is currently entered into Annex I of Council Regulation (EEC) No. 2377/90 in accordance with the following table: Pharmacologically Marker residue Animal MRLs Target Other active substance(s) species tissues provisions Albendazole Sum of albendazole Bovine, 100 µg/kg Muscle sulphoxide, ovine 100 µg/kg Fat albendazole sulphone 1000 µg/kg Liver and albendazole 2- 500 µg/kg Kidney amino sulphone 100 µg/kg Milk expressed as albendazole 2. In reviewing the availability of endo- and ectoparasiticides for sheep and goats, albendazole was considered for extrapolation from bovine and ovine species to all ruminants. The considerations and criteria leading to the identification of albendazole are described in the Position Paper Regarding Availability of Veterinary Medicines – Extrapolation of MRLs (EMEA/CVMP/457/03-FINAL). 3. The scientific justification for this extrapolation was assessed in accordance with the Notes for Guidance on Risk Analysis Approach for Residues of Veterinary Medicinal Products in Food of Animal Origin (EMEA/CVMP/187/00-FINAL) and on the Establishment of Maximum Residue Limits for Minor Animal Species (EMEA/CVMP/153a/97-FINAL). 4. In setting the ADI in the original assessment of albendazole, the data summarised on the paragraphs below were considered. 5. The mode of action of albendazole is by binding strongly with the tubulin in the cells of nematodes. -

Efficacy and Tolerability of Quinacrine Monotherapy and Albendazole Plus Chloroquine Combination Therapy in Nitroimidazole-Refractory Giardiasis: a Tropnet Study
Klinik für Infektiologie & Spitalhygiene Efficacy and tolerability of quinacrine monotherapy and albendazole plus chloroquine combination therapy in nitroimidazole-refractory giardiasis: a TropNet study Andreas Neumayr, Mirjam Schunk, Caroline Theunissen, Marjan Van Esbroeck, Matthieu Mechain, Manuel Jesús Soriano Pérez, Kristine Mørch, Peter Sothmann, Esther Künzli, Camilla Rothe, Emmanuel Bottieau Journal Club 01.03.21 Andreas Neumayr Background on giardia treatment: • 1st-line treatment: 5-nitroimidazoles: metronidazole (1957), tinidazole, ornidazole, secnidazole • cure rate of 5NIs in 1st-line treatment: ~90% • in the last decade, an increase of 5NI-refractory giardia cases has been observed in travel medicine clinics across Europe: Hospital for Tropical Diseases, London: 2008: 15% --> 2013: 40% 70% of 5NI-refractory cases imported from India • 2nd-line treatment: effectiveness of a 2nd round with a 5NI: ~17% alternative drugs: albendazole, mebendazole, nitazoxanide, quinacrine, furazolidone, chloroquine, paromomycin 2012 TropNet member survey: 53 centres use 39 different treatment regimens, consisting of 7 different drugs in mono- or combination-therapy in various dosages and durations JC 01.03.21 Nabarro LE et al. Clin Microbiol Infect. 2015;21:791-6. • by 2013, there were only 13 reports of 2nd-line therapy for giardiasis (8 case series, 5 individual case reports): n=110 Cure rates Albendazole 6/32 18.7% Paromomycin 5/17 29.4% Nitazoxanide 2/5 40.0% Albendazole + 5-NI 42/53 79.2% Quinacrine 19/21 90.5% Quinacrine + 5-NI 14/14 100% Quinacrine + Paromomycin 2/2 100% • 2013: TropNet "GiardiaREF" study kick-off: Study on efficacy and tolerability of two 2nd-line regimens in nitroimidazole-refractory giardiasis: Quinacrine JC 01.03.21 Meltzer E et al. -

204684Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 204684Orig1s000 OFFICE DIRECTOR MEMO Deputy Office Director Decisional Memo Page 2 of 17 NDA 204,684 Miltefosine Capsules 1. Introduction Leishmania organisms are intracellular protozoan parasites that are transmitted to a mammalian host by the bite of the female phlebotomine sandfly. The main clinical syndromes are visceral leishmaniasis (VL), cutaneous leishmaniasis (CL), and mucosal leishmaniasis (ML). VL is the result of systemic infection and is progressive over months or years. Clinical manifestations include fever, hepatomegaly, splenomegaly, and bone marrow involvement with pancytopenia. VL is fatal if untreated. Liposomal amphotericin B (AmBisome®) was FDA approved in 1997 for the treatment of VL. CL usually presents as one or more skin ulcers at the site of the sandfly bite. In most cases, the ulcer spontaneously resolves within several months, leaving a scar. The goals of therapy are to accelerate healing, decrease morbidity and decrease the risk of relapse, local dissemination, or mucosal dissemination. There are no FDA approved drugs for the treatment of CL. Rarely, CL disseminates from the skin to the naso-oropharyngeal mucosa, resulting in ML. ML can also develop some time after CL spontaneous ulcer healing. The risk of ML is thought to be highest with CL caused by the subgenus Viannia. ML is characterized in the medical literature as progressive with destruction of nasal and pharyngeal structures, and death may occur due to complicating aspiration pneumonia. There are no FDA approved drugs for the treatment of ML. Paladin Therapeutics submitted NDA 204, 684 seeking approval of miltefosine for the treatment of VL caused by L. -

Antipyretic Efficacy of an Initial 30-Mg/Kg Loading Dose of Acetaminophen Versus a 15-Mg/Kg Maintenance Dose
Antipyretic Efficacy of an Initial 30-mg/kg Loading Dose of Acetaminophen Versus a 15-mg/kg Maintenance Dose Jean Marc Tre´luyer, MD, PhD*; Sylvie Tonnelier, PharmD*; Philippe d’Athis‡; Beatrice Leclerc§; Isabelle Jolivet-Landreau, MD§; and Ge´rard Pons, MD, PhD* ABSTRACT. Objective. To compare the antipyretic comfort.1 Among the available antipyretic agents, efficacy of an initial 30-mg/kg acetaminophen loading acetaminophen is one of the most widely used be- dose versus a 15-mg/kg maintenance dose. cause it has an antipyretic efficacy equivalent to the Methods. A double-blind, parallel-group, random- other antipyretic drugs and a good safety profile.2–4 ized clinical trial was conducted. A total of 121 febrile Acetaminophen antipyretic efficacy is dose-depen- (rectal temperature between 39°C and 40°C) but other- dent.5,6 The recommended dose is 15 mg/kg every 6 wise healthy outpatients who were 4 months to 9 years of 2 age and weighed 4 to 26 kg were assigned randomly to 1 hours. Following this dosing schedule, it usually -takes several hours after the first acetaminophen ad ؍ and 30 mg/kg (n (62 ؍ of the dose groups: 15 mg/kg (n 59). ministration to obtain the maximal clinical efficacy of Results. In an “intention to treat” analysis, the time to the drug.7 Therefore, Mahar et al8 suggested a phys- obtain a temperature lower than 38.5°C was significantly ical “cold therapy” at the initiation of the chemical shorter in the 30-mg/kg than in the 15-mg/kg group treatment to decrease the time course to antipyretic minutes vs 139 ؎ 113 minutes). -

Antiemetics/Antivertigo Agents
Antiemetic Agents Therapeutic Class Review (TCR) May 1, 2019 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. May 2019 Proprietary Information. Restricted Access – Do not disseminate or copy without approval. © 2004-2019 Magellan Rx Management. All Rights Reserved. 3 FDA-APPROVED INDICATIONS Drug Manufacturer Indication(s) NK1 receptor antagonists aprepitant capsules generic, Merck In combination with other antiemetic agents for: (Emend®)1 . -

Nitrous Oxide in Emergency Medicine Í O’ Sullivan, J Benger
214 ANALGESIA Emerg Med J: first published as 10.1136/emj.20.3.214 on 1 May 2003. Downloaded from Nitrous oxide in emergency medicine Í O’ Sullivan, J Benger ............................................................................................................................. Emerg Med J 2003;20:214–217 Safe and predictable analgesia is required for the identify these zones as there is considerable vari- potentially painful or uncomfortable procedures often ation between people. He also emphasised the importance of the patient’s pre-existing beliefs. If undertaken in an emergency department. The volunteers expect to fall asleep while inhaling characteristics of an ideal analgesic agent are safety, 30% N2O then a high proportion do so. An appro- predictability, non-invasive delivery, freedom from side priate physical and psychological environment increases the actions of N2O and may allow lower effects, simplicity of use, and a rapid onset and offset. doses to be more effective. Unlike many other Newer approaches have threatened the widespread use anaesthetic agents, N2O exhibits an acute toler- of nitrous oxide, but despite its long history this simple ance effect, whereby its potency is greater at induction than after a period of “accommoda- gas still has much to offer. tion”. .......................................................................... MECHANISM OF ACTION “I am sure the air in heaven must be this Some writers have suggested that N2O, like wonder-working gas of delight”. volatile anaesthetics, causes non-specific central nervous system depression. Others, such as 4 Robert Southey, Poet (1774 to 1843) Gillman, propose that N2O acts specifically by interacting with the endogenous opioid system. HISTORY N2O is known to act preferentially on areas of the Nitrous oxide (N2O) is the oldest known anaes- brain and spinal cord that are rich in morphine thetic agent. -

Analgesic Indications: Developing Drug and Biological Products
Guidance for Industry Analgesic Indications: Developing Drug and Biological Products DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document should be submitted within 60 days of publication in the Federal Register of the notice announcing the availability of the draft guidance. Submit electronic comments to http://www.regulations.gov. Submit written comments to the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD 20852. All comments should be identified with the docket number listed in the notice of availability that publishes in the Federal Register. For questions regarding this draft document contact Sharon Hertz at 301-796-2280. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2014 Clinical/Medical 5150dft.doc 01/15/14 Guidance for Industry Analgesic Indications: Developing Drug and Biological Products Additional copies available from: Office of Communications, Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration 10903 New Hampshire Ave., Bldg. 51, rm. 2201 Silver Spring, MD 20993-0002 Tel: 301-796-3400; Fax: 301-847-8714; E-mail: [email protected] http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February