Issues of the Presence of Parasitic Protozoa in Surface Waters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Primary Amoebic Meningoencephalitis Amoebic Meningoencephalitis Is Primary Ś
PØEHLEDOVÉ PRÁCE PØEHLEDOVÉ JE NEGLERIÓZA VEREJNO-ZDRAVOTNÍCKYM PROBLÉMOM? IS PRIMARY AMOEBIC MENINGOENCEPHALITIS (NAEGLERIASIS) A PUBLIC HEALTH PROBLEM? KATARÍNA TRNKOVÁ, LUCIA MAĎAROVÁ, CYRIL KLEMENT Regionálny úrad verejného zdravotníctva so sídlom v Banskej Bystrici, odbor lekárskej mikrobiológie SOUHRN Neglerióza alebo primárna amébová meningoencefalitída (PAM) je zriedkavé ochorenie CNS, pôvodcom ktorého je vo¾ne žijúca meòavka Naegleria fowleri. Medzi stovkami vo¾ne žijúcich meòaviek sú známe i ïalšie rody, ktorých zástupcovia sú schopní infikovaś èloveka a vyvolaś u neho ochorenie. Za patogény sú považovaní zástupcovia rodov Acanthamoeba a Naegleria a druhy Balamuthia mandrillaris a Sappi- nia diploidea. Infekcie spôsobené týmito organizmami vyvolávajú u ¾udí syndrómy v rozsahu od akútnych fatálnych ochorení po chronické, tkanivá napadajúce infekcie s granulomatóznymi prejavmi. Epidemiológia, imunológia, patológia a klinické prejavy týchto infekcií sa vzájomne ve¾mi líšia. Príspevok podáva preh¾ad o pôvodcovi ochorenia PAM, o jeho morfológii, životnom cykle, ekológii ako aj o patogenéze, symptomatike a spôsoboch laboratórnej diagnostiky negleriózy. K¾úèové slová: neglerióza, primárna amébová meningoencefalitída, epidemiológia, laboratórna diagnostika Naegleria fowleri SUMMARY Naegleriasis or primary amoebic meningoencephalitis (PAM) is invariably an acute, often fulminant infection of CNS caused by Naegleria fowleri, a small, free-living amoeba. Pathogenic free-living amoebae can cause serious illnesses in humans. The amoe- HYGIENA bae belonging to the genus Naegleria, Acanthamoeba and Balamuthia mandrillaris and Sappinia diploidea produce syndromes in man ranging from acute fatal disease to chronic tissue invasion with granulomatous manifestation. The purpose of this report is to describe the clinical history, treatment, pathology and methods of laboratory diagnostic of naegleriasis. Key words: primary amoebic meningoencephalitis, naegleriasis, epidemiology, laboratory diagnostics of Naegleria fowleri ÈÍSLO 2 Úvod Obr. -

SNF Mobility Model: ICD-10 HCC Crosswalk, V. 3.0.1
The mapping below corresponds to NQF #2634 and NQF #2636. HCC # ICD-10 Code ICD-10 Code Category This is a filter ceThis is a filter cellThis is a filter cell 3 A0101 Typhoid meningitis 3 A0221 Salmonella meningitis 3 A066 Amebic brain abscess 3 A170 Tuberculous meningitis 3 A171 Meningeal tuberculoma 3 A1781 Tuberculoma of brain and spinal cord 3 A1782 Tuberculous meningoencephalitis 3 A1783 Tuberculous neuritis 3 A1789 Other tuberculosis of nervous system 3 A179 Tuberculosis of nervous system, unspecified 3 A203 Plague meningitis 3 A2781 Aseptic meningitis in leptospirosis 3 A3211 Listerial meningitis 3 A3212 Listerial meningoencephalitis 3 A34 Obstetrical tetanus 3 A35 Other tetanus 3 A390 Meningococcal meningitis 3 A3981 Meningococcal encephalitis 3 A4281 Actinomycotic meningitis 3 A4282 Actinomycotic encephalitis 3 A5040 Late congenital neurosyphilis, unspecified 3 A5041 Late congenital syphilitic meningitis 3 A5042 Late congenital syphilitic encephalitis 3 A5043 Late congenital syphilitic polyneuropathy 3 A5044 Late congenital syphilitic optic nerve atrophy 3 A5045 Juvenile general paresis 3 A5049 Other late congenital neurosyphilis 3 A5141 Secondary syphilitic meningitis 3 A5210 Symptomatic neurosyphilis, unspecified 3 A5211 Tabes dorsalis 3 A5212 Other cerebrospinal syphilis 3 A5213 Late syphilitic meningitis 3 A5214 Late syphilitic encephalitis 3 A5215 Late syphilitic neuropathy 3 A5216 Charcot's arthropathy (tabetic) 3 A5217 General paresis 3 A5219 Other symptomatic neurosyphilis 3 A522 Asymptomatic neurosyphilis 3 A523 Neurosyphilis, -

Toothless Aquatic Code Allows Deadly, Brain-Eating Zombie Amoeba to Flourish in Arizona Splash Pads and Water Playgrounds Sarah Pook*
IF WE ONLY HAD A BRAIN: Toothless Aquatic Code Allows Deadly, Brain-Eating Zombie Amoeba To Flourish in Arizona Splash Pads and Water Playgrounds Sarah Pook* I. INTRODUCTION It starts with a fever. A splitting headache. Vomiting, fatigue, an earache— then the secondary symptoms begin. Vision loss. Stiff neck. Lethargy, confusion, inability to walk, an aversion to light. Hallucinations. Doctors scramble to make a diagnosis, attempting treatment for bacterial meningitis, viral encephalitis, herpes, or other rare diseases, but nothing works.1 Finally, a coma. Death follows within three days.2 Diagnosis is usually done post-mortem: the culprit is primary amebic meningoencephalitis, or PAM.3 The disease is identified via a cerebrospinal fluid tap under a microscope. Peering in, you can see free living amoeba swimming around in the spinal fluid.4 * J.D. Candidate, 2020, Sandra Day O’Connor College of Law, Arizona State University; Editor-in-Chief, Arizona State Law Journal. I would like to thank Professor Tamara Herrera at Arizona State University for her insight and never-ending support as my faculty advisor, and Professor Marsha Howard at the University of Tulsa for her introduction to this fascinating topic. I would also like to thank my family for their love and encouragement. Finally, my heartfelt thanks to the wonderful Arizona State Law Journal staff and editors who bring our issues to publication—what a joy it is to work with all of you! 1. See Rikesh Baral & Binit Vaidya, Fatal Case of Amoebic Encephalitis Masquerading as Herpes, 5 OXFORD MED. CASE REPORTS. 146, 148 (2018), https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5934662/pdf/omy010.pdf [https://perma.cc/HSM6-WZZV]. -

Taxonomy of Clinically Relevant Microorganisms*
OpenStax-CNX module: m58949 1 Taxonomy of Clinically Relevant Microorganisms* OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 1 Bacterial Pathogens The following tables list the species, and some higher groups, of pathogenic Eubacteria mentioned in the text. The classication of Bacteria, one of the three domains of life, is in constant ux as relationships become clearer through sampling of genetic sequences. Many groups at all taxonomic levels still have an undetermined relationship with other members of the phylogenetic tree of Bacteria. Bergey's Manual of Systematics of Archaea and Bacteria maintains a published list and descriptions of prokaryotic species. The tables here follow the taxonomic organization in the Bergey's Manual Taxonomic Outline.1 We have divided the species into tables corresponding to dierent bacterial phyla. The taxonomic rank of kingdom is not used in prokaryote taxonomy, so the phyla are the subgrouping below domain. Note that many bacterial phyla not represented by these tables. The species and genera are listed only under the class within each phylum. The names given to bacteria are regulated by the International Code of Nomenclature of Bacteria as maintained by the International Committee on Systematics or Prokaryotes. Phylum Actinobacteria Class Genus Species Related Diseases Corynebacterium diphtheriae Diphtheria Gardnerella vaginalis Bacterial vaginosis Micrococcus Opportunistic infections Actinobacteria Mycobacterium bovis Tuberculosis, primarily in cattle Mycobacterium leprae Hansen's disease continued on next page *Version 1.5: Apr 4, 2018 1:24 pm -0500 http://creativecommons.org/licenses/by/4.0/ 1Bergey's Manual Trust. Bergey's Manual of Systematics of Archaea and Bacteria, Taxonomic Outline. -

Is This an Outbreak?
Is This an Outbreak? A retrospective evaluation of syndromic surveillance for emerging infectious disease Is This an Outbreak? A retrospective evaluation of syndromic surveillance for emerging infectious disease Is dit een uitbraak? Een retrospectieve evaluatie van syndroomsurveillance voor nieuw opduikende infectieziekten Proefschrift ter verkrijging van de graad van doctor aan de Erasmus Universiteit Rotterdam op gezag van de rector magnificus Prof.dr. H.G. Schmidt en volgens besluit van het College voor Promoties. De openbare verdediging zal plaatsvinden op donderdag 9 december 2010 om 13.30 uur door © 2010 Kees van den Wijngaard, Zeist Cornelis Carolus van den Wijngaard ISBN 978 90 6464 426 9 Layout: GVO drukkers & vormgevers B.V. | Ponsen & Looijen geboren te Sittard Printed by: GVO drukkers & vormgevers B.V. | Ponsen & Looijen Reprints were made with permission of The American Public Health Association and Cambridge University Press. Publication of this thesis was financially supported by the National Institute for Public Health and the Environment (RIVM) and Erasmus University Rotterdam (EUR). Promotiecommissie Promotoren: Prof.dr. M.P.G. Koopmans Prof.dr. N.J.D. Nagelkerke Overige leden: Prof.dr. A. Osterhaus Prof.dr. J.H. Richardus Prof.dr. M.C.J.M. Sturkenboom Copromotor: Dr. W. van Pelt Contents Chapter 1 General Introduction 9 Chapter 2 Validation of Syndromic Surveillance for Respiratory Pathogen Activity Emerg Infect Dis 2008;14:917-25 19 Chapter 3 Detection of Excess Influenza Severity: Associating Respiratory Hospitalization -
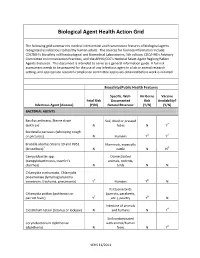
Biological Agent Health Action Grid
Biological Agent Health Action Grid The following grid summarizes medical intervention and transmission features of biological agents recognized as infectious to healthy human adults. The sources for foonote information include: CDC/NIH’s Biosafety in Microbiological and Biomedical Laboratories, 5th edition; CDC/HHS’s Advisory Committee on Immunization Practices; and the APHIS/CDC’s National Select Agent Registry/Select Agents Exclusion. This document is intended to serve as a general information guide. A full risk assessment needs to be prepared for the use of any infectious agent in a lab or animal research setting, and appropriate research compliance committee approvals obtained before work is initiated. Biosafety/Public Health Features Specific, Well- Air-Borne Vaccine Fetal Risk Documented Risk Availability? Infectious Agent (disease) (Y/N) Natural Reservoir (Y/N) (Y/N) BACTERIAL AGENTS Bacillus anthracis, Sterne strain Soil, dried or pressed (anthrax)1 N hides N Y2 Bordetella pertussis (whooping cough or pertussis) N Humans Y3 Y4 Brucella abortus Strains 19 and RB51 Mammals, especially (brucellosis)5 N cattle N N6 Campylobacter spp. Domesticated (campylobacteriosis, traveler's animals, rodents, diarrhea) N birds N N Chlamydia trachomatis, Chlamydia pneumoniae (lymphogranuloma venereum, trachoma, pneumonia) Y7 Humans Y8 N Psittacine birds Chlamydia psittaci (psittacosis or (parrots, parakeets, parrott fever) Y7 etc.), poultry Y8 N Intestine of animals Clostridium tetani (tetanus or lockjaw) N and humans N Y4 Soil contaminated Corynebacterium diphtheriae with animal/human (diphtheria) N feces N Y4 VEHS 11/2011 Specific, Well- Air-Borne Vaccine Fetal Risk Documented Risk Availability? Infectious Agent (disease) (Y/N) Natural Reservoir (Y/N) (Y/N) Francisella tularensis, subspecies Wild animals, esp. -

A Map of the Alternative Biomedical R&D Landscape
A map of the alternative biomedical R&D landscape ACKNOWLEDGEMENTS We would like to acknowledge all the engaged and active student leaders in UAEM who have supported, participated and activated around the critical global health issue of alternative biomedical R&D, the role of universities and ensuring access to medicines for all. Thanks as well as to the staff and board of UAEM. Special thanks to Els Torreele PhD, Director of the Open Society Public Health Program’s Access to Essential Medicines Initiative, for her advice, support and encouragement. And thanks to the team at signals.ca for providing us with their design talent and enthusiastically agreeing to complete this project in record time! AUTHOR BIOGRAPHIES Rachel Kiddell-Monroe LL.M is a humanitarian, a lawyer and an activist. She is Special Adviser to Universities Allied for Essential Medicines (UAEM) and served 6 years as President of the UAEM board of directors. She is also a member of the International Board of directors for Médecins Sans Frontières/Doctors Without Borders (MSF). She lives in Montreal and has worked in south east Asia with human rights and environmental grassroots activists as well as with MSF in Djibouti, Rwanda, the Democratic Republic of Congo and throughout Latin America. Rachel has also served as the Canadian director of MSF’s Access to Medicines Campaign. Rachel sits on the board of UAEM Europe, on the Advisory Council of UAEM Brazil and the board of the Young Professionals for Chronic Disease Network. Rachel is a Professor of Practice at McGill University, lectures on international development and is part of the Adult Clinical Ethics Committee at McGill University Health Centres. -

The Epidemiology of Primary Amoebic Meningoencephalitis in the USA, 1962–2008
Epidemiol. Infect. (2010), 138, 968–975. f Cambridge University Press 2009 This is a work of the U.S. Government and is not subject to copyright protection in the United States doi:10.1017/S0950268809991014 The epidemiology of primary amoebic meningoencephalitis in the USA, 1962–2008 J. S. YODER 1*, B. A. EDDY1,2,G.S.VISVESVARA1,L.CAPEWELL1 AND M. J. BEACH 1 1 Division of Parasitic Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA 2 Rollins School of Public Health, Emory University, Atlanta, GA, USA (Accepted 18 September 2009; first published online 22 October 2009) SUMMARY Naegleria fowleri, a free-living, thermophilic amoeba ubiquitous in the environment, causes primary amoebic meningoencephalitis (PAM), a rare but nearly always fatal disease of the central nervous system. While case reports of PAM have been documented worldwide, very few individuals have been diagnosed with PAM despite the vast number of people who have contact with fresh water where N. fowleri may be present. In the USA, 111 PAM case-patients have been prospectively diagnosed, reported, and verified by state health officials since 1962. Consistent with the literature, case reports reveal that N. fowleri infections occur primarily in previously healthy young males exposed to warm recreational waters, especially lakes and ponds, in warm-weather locations during summer months. The annual number of PAM case reports varied, but does not appear to be increasing over time. Because PAM is a rare disease, it is challenging to understand the environmental and host-specific factors associated with infection in order to develop science-based, risk reduction messages for swimmers. -

48Th Issue June - September 2020 SEASONAL AWARENESS and ALERT LETTER (SAAL) for Epidemic-Prone Infectious Diseases in Pakistan Summer / Monsoon Season
Ministry of Naonal Health Services, Regulaons & Coordinaon Government of Pakistan Naonal Instute of Health, Islamabad, Pakistan Field Epidemiology & Disease Surveillance Division (FE&DSD) Tel: 051-9255237, 9255575 Naonal Focal Point for Internaonal Health Regulaons (IHR) 48th Issue June - September 2020 SEASONAL AWARENESS AND ALERT LETTER (SAAL) For Epidemic-prone infectious diseases in Pakistan Summer / Monsoon Season OBJECTIVES OF SAAL Outbreak - Prone Diseases Alerts Ÿ To alert concerned health authories and professionals at all levels about the Chikungunya epidemic-prone infecous diseases in the summer/Monsoon season. Cholera (Acute watery Diarrhea) Ÿ To facilitate the preparaons for mely and efficient response to the Coronavirus disease 2019 (COVID-19) encountered alerts/outbreaks/ epidemics and thus reduce the associated Crimean Congo Hemorrhagic Fever (CCHF) morbidity and mortality. Dengue Fever DATA SOURCES Diphtheria The available naonal data collected during 2015 to May 2020 by FE&DSD, NIH, Leishmaniasis Provincial Health Departments, Provincial Disease Surveillance & Response Units Malaria (PDSRUs), Expanded Program on Immunizaon (EPI), Directorate of Malaria Control and laboratory based data from NIH has been analyzed to assess the Measles exhibited paerns of high priority communicable diseases. Meningococcal Meningis The descripon of all priority diseases has been arranged in an alphabecal order. Pertussis Addionally, under the secon of Naonal Potenal Public Health Events, Poliomyelis technical detail on the Heat stroke and -
Investigating the Potential for Environmental Perception and Adaptation in the Amoebaflagellate Naegleria Gruberi
University of Huddersfield Repository Sykes, Connor Investigating the potential for environmental perception and adaptation in the amoebaflagellate Naegleria gruberi Original Citation Sykes, Connor (2019) Investigating the potential for environmental perception and adaptation in the amoebaflagellate Naegleria gruberi. Masters thesis, University of Huddersfield. This version is available at http://eprints.hud.ac.uk/id/eprint/34975/ The University Repository is a digital collection of the research output of the University, available on Open Access. Copyright and Moral Rights for the items on this site are retained by the individual author and/or other copyright owners. Users may access full items free of charge; copies of full text items generally can be reproduced, displayed or performed and given to third parties in any format or medium for personal research or study, educational or not-for-profit purposes without prior permission or charge, provided: • The authors, title and full bibliographic details is credited in any copy; • A hyperlink and/or URL is included for the original metadata page; and • The content is not changed in any way. For more information, including our policy and submission procedure, please contact the Repository Team at: [email protected]. http://eprints.hud.ac.uk/ C.Sykes U1363969 Master of Science by Research Investigating the potential for environmental perception and adaptation in the amoebaflagellate Naegleria gruberi Connor Sykes U1363969 A thesis submitted to the University of Huddersfield in partial fulfilment of the requirements for the degree of Master of Science by Research. Division of Biological Sciences & Nutrition, School of Applied Sciences, University of Huddersfield January 2019 1 C.Sykes U1363969 Master of Science by Research Acknowledgements First and foremost, I would like to express my deepest gratitude to my research supervisors, Professor Michael Ginger and Dr. -

Review on Types of Pathogens
Review https://www.alliedacademies.org/public-health-policy-planning/ Review on types of pathogens. Laurier Elod* Department of Applied Medicine, Stanford University, California, United States Accepted on July 13, 2021 Algae Parasitic Diseases Green growth are single-celled eukaryotes that are by and Protozoans are single-celled eukaryotes that feed on large non-pathogenic albeit pathogenic assortments do exist. microorganisms and natural tissues. Considered as "one-celled Protothecosis is an infection found in canines, felines, cows, and creature" as they have creature like practices like motility, people brought about by a sort of green alga known as prototheca predation, and an absence of a cell divider. Numerous protozoan that needs chlorophyll.One of the bacterial sicknesses with the microbes are viewed as human parasites as they cause an most noteworthy illness trouble is tuberculosis, brought about assortment of infections, for example, jungle fever, amoebiasis, by the bacterium Mycobacterium tuberculosis, which killed 1.5 giardiasis, toxoplasmosis, cryptosporidiosis, trichomoniasis, million individuals in 2013, generally in sub-Saharan Africa. Chagas illness, leishmaniasis, African trypanosomiasis (resting Pathogenic microbes add to other worldwide huge sicknesses, affliction), Acanthamoeba keratitis, and essential amoebic like pneumonia, which can be brought about by microscopic meningoencephalitis (naegleriasis).Parasitic worms (Helminths) organisms like Streptococcus and Pseudomonas, and foodborne are macroparasites that can -

World Influence of Infectious Diseases from Wikipedia Network Analysis
bioRxiv preprint doi: https://doi.org/10.1101/424465; this version posted September 24, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Manuscript SUBMITTED TO eLife WORLD INflUENCE OF INFECTIOUS 1 Diseases FROM Wikipedia Network 2 Analysis 3 1 1* 2* 4 Guillaume Rollin , José Lages , Dima L. Shepelyansky *For CORRespondence: INSTITUT UTINAM, ObservatoirE DES Sciences DE L’Univers THETA, CNRS, Université DE [email protected] (JL); 5 1 [email protected] (DLS) BourGOGNE Franche-Comté, 25030 Besançon, France; LaborATOIRE DE Physique 6 2 Théorique, IRSAMC, Université DE Toulouse, CNRS, UPS, 31062 Toulouse, FrANCE 7 8 9 AbstrACT WE CONSIDER THE NETWORK OF 5 416 537 ARTICLES OF English Wikipedia EXTRACTED IN 2017. 10 Using THE RECENT REDUCED Google MATRIX (REGOMAX) METHOD WE CONSTRUCT THE REDUCED NETWORK OF 11 230 ARTICLES (nodes) OF INFECTIOUS DISEASES AND 195 ARTICLES OF WORLD countries. This METHOD 12 GENERATES THE REDUCED DIRECTED NETWORK BETWEEN ALL 425 NODES TAKING INTO ACCOUNT ALL DIRECT AND 13 INDIRECT LINKS WITH PATHWAYS VIA THE HUGE GLOBAL network. PageRank AND CheiRank ALGORITHMS ARE 14 USED TO DETERMINE THE MOST INflUENTIAL DISEASES WITH THE TOP PageRank DISEASES BEING TUBERculosis, 15 HIV/AIDS AND Malaria. FrOM THE REDUCED Google MATRIX WE DETERMINE THE SENSITIVITY OF WORLD 16 COUNTRIES TO SPECIfiC DISEASES INTEGRATING THEIR INflUENCE OVER ALL THEIR HISTORY INCLUDING THE TIMES OF 17 ANCIENT Egyptian mummies.