Use of the Non-Aldol Aldol Process in the Synthesis of the C1–C11 Fragment of the Tedanolides: Use of Lactol Ethers in Place of Tetrahydrofurans
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Studies Toward Synthesis of Polycyclic Polyprenylated Acylphloroglucinols
University of Kentucky UKnowledge University of Kentucky Doctoral Dissertations Graduate School 2006 STUDIES TOWARD SYNTHESIS OF POLYCYCLIC POLYPRENYLATED ACYLPHLOROGLUCINOLS Roxana Ciochina University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Ciochina, Roxana, "STUDIES TOWARD SYNTHESIS OF POLYCYCLIC POLYPRENYLATED ACYLPHLOROGLUCINOLS" (2006). University of Kentucky Doctoral Dissertations. 291. https://uknowledge.uky.edu/gradschool_diss/291 This Dissertation is brought to you for free and open access by the Graduate School at UKnowledge. It has been accepted for inclusion in University of Kentucky Doctoral Dissertations by an authorized administrator of UKnowledge. For more information, please contact [email protected]. ABSTRACT OF DISSERTATION Roxana Ciochina The Graduate School University of Kentucky 2006 STUDIES TOWARD SYNTHESIS OF POLYCYCLIC POLYPRENYLATED ACYLPHLOROGLUCINOLS ABSTRACT OF DISSERTATION A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the College of Arts and Sciences at the University of Kentucky By Roxana Ciochina Lexington, KY Director: Dr. R. B. Grossman, Professor of Chemistry Lexington, KY 2006 ABSTRACT OF DISSERTATION STUDIES TOWARD SYNTHESIS OF POLYCYCLIC POLYPRENYLATED ACYLPHLOROGLUCINOLS Polycyclic polyprenylated acylphloroglucinols (PPAPs) are a class of compounds that reveal intriguing biological activities and interesting and challenging chemical structures. These products are claimed to possess antioxidant, antiviral, and antimitotic properties. Increasing interest is related to their function in the CNS as modulators of neurotransmitters associated to neuronal damaging and depression. All these features make PPAPs targets for synthesis. We decided to focus our own initial efforts in this area on the type A PPAP, nemorosone because we thought that its fairly simple structure relative to other PPAPs would present fewer hurdles as we developed our methodology. -

Synthesis of Alkynyl Ribofuranosides
City University of New York (CUNY) CUNY Academic Works Dissertations and Theses City College of New York 2011 Synthesis of Alkynyl Ribofuranosides Christian Rodriguez CUNY City College How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/cc_etds_theses/25 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] SYNTHESIS OF ALKYNYL RIBOFURANOSIDES A Thesis Presented to The Faculty of the Chemistry Program The City College of New York In (Partial) Fulfillment of the Requirements for the Degree Master of Arts by Christian Rodriguez December, 2010 - 1 - Synthesis of Alkynyl Ribofuranosides By Christian Rodriguez Mentor: P. Meleties Table of Contents Chapter 1 1.1 Introduction 6 1.2 Preparation of ribonolactone template 8 1.3 Synthesis of protected ribonolactone 9 Chapter 2 2.1 Preparation of ethynyl ribofuranosides 10 2.2 Reaction with ethynylmagnesium bromide 12 2.3 Intramolecular cyclization of diyne diol 14 2.4 Reaction of ribonolactone with lithium acetylide 15 2.5 Boron trifluoride hemiacetal deoxygenation 15 2.6 Alternative deacetylation 16 Chapter 3 3.1 Selecting appropriate protecting group 19 3.2 Preparation of trimethylsilyl alkynyl 5-O-benzyl-2,3-O isopropylidene 19 ribofuranoside 3.3 Lewis acid promoted triethylsilane dehydroxylation mechanism 20 Chapter 4 4.1 Hemiacetal alkynylation 25 4.2 Hemiacetal nucleophilic addition mechanism 26 4.3 Intramolecular -
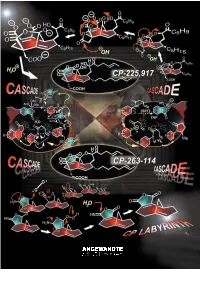
A Paradigm of How Endeavors in Total Synthesis Lead to Discoveries and Inventions in Organic Synthesis
REVIEWS The CP Molecule Labyrinth: A Paradigm of How Endeavors in Total Synthesis Lead to Discoveries and Inventions in Organic Synthesis K. C. Nicolaou* and Phil S. Baran Dedicated to Mrs. Niki Goulandris for her outstanding contributions to humanity and Planet Earth on the occasion of the opening of the GAIA Center for Environmental Research and Education at the Goulandris Natural History Museum in Athens, Greece. Imagine an artist carving a sculpture Herculean nature of the task and the ed Minotaur, which he accomplished from a marble slab and finding gold rewards that accompany it, one must through brilliance, skill, and bravery nuggets in the process. This thought is sense the details of the enterprise having traversed the famous labyrinth not a far-fetched description of the behind the scenes. A more vivid de- with the help of Ariadne. This story work of a synthetic chemist pursuing scription of total synthesis as a struggle from Greek mythology comes alive in the total synthesis of a natural product. against a tough opponent is perhaps modern synthetic expeditions toward At the end of the day, he or she will be appropriate to dramatize these ele- natural products as exemplified by the judged by the artistry of the final work ments of the experience. In this article total synthesis of the CP molecules and the weight of the gold discovered we describe one such endeavor of total which serve as a paradigm for modern in the process. However, as colorful as synthesis which, in addition to reaching total synthesis endeavors, where the this description of total synthesis may the target molecule, resulted in a objectives are discovery and invention be, it does not entirely capture the wealth of new synthetic strategies and in the broader sense of organic syn- essence of the endeavor, for there is technologies for chemical synthesis. -

Application of Complex Aldol Reactions to the Total Synthesis of Phorboxazole B
J. Am. Chem. Soc. 2000, 122, 10033-10046 10033 Application of Complex Aldol Reactions to the Total Synthesis of Phorboxazole B David A. Evans,* Duke M. Fitch,1 Thomas E. Smith, and Victor J. Cee Contribution from the Department of Chemistry and Chemical Biology, HarVard UniVersity, Cambridge, Massachusetts 02138 ReceiVed June 29, 2000 Abstract: The synthesis of phorboxazole B has been accomplished in 27 linear steps and an overall yield of 12.6%. The absolute stereochemistry of the C4-C12,C33-C38, and C13-C19 fragments was established utilizing catalytic asymmetric aldol methodology, while the absolute stereochemistry of the C20-C32 fragment was derived from an auxiliary-based asymmetric aldol reaction. All remaining chirality was incorporated through internal asymmetric induction, with the exception of the C43 stereocenter which was derived from (R)-trityl glycidol. Key fragment couplings include a stereoselective double stereodifferentiating aldol reaction, a metalated oxazole alkylation, an oxazole-stabilized Wittig olefination, and a chelation-controlled addition of the fully elaborated alkenyl metal side chain. Introduction and HT29 (3.31 × 10-10 M), as well as leukemia CCRF-CBM (2.45 × 10-10 M), prostate cancer PC-3 (3.54 × 10-10 M), and Phorboxazoles A (1)andB(2) are marine natural products breast cancer MCF7 cell lines (5.62 × 10-10 M).2b In addition isolated from a recently discovered species of Indian Ocean to this impressive anticancer activity, phorboxazoles A and B sponge (genus Phorbas sp.) near Muiron Island, Western also exhibit potent in vitro antifungal activity against Candida Australia.2 These substances are representative of a new class albicans and Saccharomyces carlsbergensis at 0.1 µg/disk in of macrolides differing only in their C hydroxyl-bearing 13 the agar disk diffusion assay.2a stereocenters. -

II Reduction Reactions
II Reduction Reactions Objectives By the end of this section you will: 1) be able to exploit the differences in reactivity of various reducing agents (hydride vs neutral reductants) in chemoselective reductions and be able to provide a mechanistic rationale to account for their differing reactivities; 2) be able to use the inherent chirality in a substrate to control the outcome of a reduction of proximal ketones to generate selectively syn and anti 1,3- and 1,2-diols; 3) be able to rationalise the outcome of these diastereoselective reactions using T.S. diagrams; 4) have gained an appreciation of the versatility of transition metals in reduction reactions; 5) have gained an appreciation of the synthetic utility of dissolving metal reductions; 6) be able to use radical chemistry for deoxygenation and reduction of halides. II.A Reduction of Carboxylic Acid Derivatives and Related Functionality OR' H ROH RO RO carboxylic acid aldehyde primary alcohol derivatives R N RNH2 RNO2 Issues of Reactivity and Selectivity Similar issues of selectivity and reactivity to those we encountered in the case of oxidation reactions also arise in reduction reactions. 1. Chemoselectivity. Many different functional groups can be reduced in a variety of ways. We often need to selectively reduce one functional group whilst leaving others intact (remember year 1 practical!). NaBH4 Sn, HCl OH O O O2N O2N H2N Chemoselective reductions from a practical in CHM1C3 2. In the case of carboxylic acid derivatives there are two possible reduction products: an aldehyde and an alcohol. Ideally we need methods for selectively accessing either product. -

Studies on the Prebiotic Origin of 2-Deoxy-D-Ribose
Studies on the Prebiotic Origin of 2-Deoxy-D-ribose Andrew Mark Steer Doctor of Philosophy University of York Chemistry August 2017 Abstract DNA is an important biological structure necessary for cell proliferation. The origins of cell- like structures and the building blocks of DNA are therefore also of great concern. As of yet the prebiotic origin of 2-deoxy-D-ribose, the sugar of DNA, has no satisfactory explanation. This research attempts to provide a possible explanation to the chemical origin of 2-deoxy- D-ribose via an aldol reaction between acetaldehyde 1 and D-glyceraldehyde D-2 (Error! Reference source not found.). The sugar mixture is trapped with N,N-diphenylhydrazine 3 for ease of purification and characterisation. The reaction is promoted by amino acids, amino esters and amino nitriles consistently giving selectivities in favour of 2-deoxy-D- ribose. This is the first example of an amino nitrile promoted reaction. Potential prebiotic synthesis of 2-deoxy-D-ribose and subsequent trapping with N,N-diphenyl hydrazine 3. The research is developed further by exploring the formation of 2-deoxy-D-ribose in a “protocell” environment – a primitive cell. Here we suggest that primitive cells may have been simple hydrogel systems. A discussion of the characterisation and catalytic ability of small peptide-based supramolecular structures is included. ii Contents Abstract ............................................................................................................................ ii Contents ......................................................................................................................... -

Efforts Towards the Total Synthesis of Azaspiracid-3 DISSERTATION
Efforts towards the Total Synthesis of Azaspiracid-3 DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Zhigao Zhang Graduate Program in Chemistry The Ohio State University 2013 Dissertation Committee: Dr. Craig J. Forsyth, Advisor Dr. Jon R. Parquette Dr Anita E. Mattson Copyright by Zhigao Zhang 2013 Abstract Azaspiracid, a structurally complex marine toxin isolated from the mussel Mytilus edulis in Killary Harbor, Ireland, represents a new class of marine metabolites unrelated to any previous known agent of diarrhetic shellfish posioning. As such, the natural product has spurred considerable interest among organic community. The thesis details the study towards the total synthesis of azaspiracid-3. The C1-C21 domain and C22-C40 domain have been synthesized, which aims to assemble the molecule through Yamaguchi esterification. The ABCD domain involved an efficient NHK coupling reaction between C1-C12 segment and C13-C22 segment. The trioxadispiroketal skeleton was constructed under the influence of acid catalysis. The EFGHI domain highlighted a chelate controlled Mukaiyama aldol reaction to produce C22-C40 linear precursor, and a DIHMA process to assemble the bridged ketal. With the recognition of bridged ketal and spiroaminal functionality in the FGHI domain, the gold-catalysis to achieve these moieties has been proposed. A novel gold- catalyzed spiroaminal formation has been systemically developed. The gold-catalyzed ketallization was then successfully implemented to construct the bridged ketal, while an inevitable elimination could not deliver the desired spiroaminal under gold conditions. A NHK reaction to couple the C1-C21 and C22-C40 domains has also been proposed. -

Nucleophilic Addition to the Carbonyl Group 6
Nucleophilic addition to the carbonyl group 6 Connections Building on Arriving at Looking forward to • Functional groups, especially the C=O • How and why the C=O group reacts • Additions of organometallic group ch2 with nucleophiles reagents ch9 • Identifying the functional groups in a • Explaining the reactivity of the C=O • Substitution reactions of the C=O molecule spectroscopically ch3 group using molecular orbitals and curly group’s oxygen atom ch11 • How molecular orbitals explain arrows • How the C=O group in derivatives of molecular shapes and functional • What sorts of molecules can be made by carboxylic acids promotes substitution groups ch4 reactions of C=O groups reactions ch10 • How, and why, molecules react together • How acid or base catalysts improve the • C=O groups with an adjacent double and using curly arrows to describe reactivity of the C=O group bond ch22 reactions ch5 Molecular orbitals explain the reactivity of the carbonyl group We are now going to leave to one side most of the reactions you met in the last chapter—we will come back to them all again later in the book. In this chapter we are going to concentrate on just one of them—probably the simplest of all organic reactions—the addition of a nucleo- phile to a carbonyl group. The carbonyl group, as found in aldehydes, ketones, and many other compounds, is without doubt the most important functional group in organic chemis- try, and that is another reason why we have chosen it as our fi rst topic for more detailed study. You met nucleophilic addition to a carbonyl group on pp. -

Organic & Biomolecular Chemistry
Organic & Biomolecular Chemistry Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/obc Page 1 of 16 OrganicPlease do not & Biomolecularadjust margins Chemistry Organic & Biomolecular Chemistry REVIEW Alkaloid Synthesis using Chiral Secondary Amine Organocatalysts Received 00th January 20xx, Hayato Ishikawa,* and Shinya Shiomi Accepted 00th January 20xx Manuscript DOI: 10.1039/x0xx00000x Over the last decade, several excellent enantioselective total syntheses of important alkaloids using asymmetric reactions mediated by chiral secondary amine organocatalysts as a key step have been accomplished. This perspective article www.rsc.org/ examines the full strategies of these alkaloid syntheses, especially the application of the organocatalytic reaction to construct the alkaloid scaffolds. catalyst),4 have been used to catalyze several important Introduction enantioselective reactions, such as asymmetric aldol, Michael, Mannich, Diels–Alder and ene-reactions, as well as α- Alkaloids containing a basic amine portion in the molecule are oxidations, and epoxidations (Fig. -

Download Author Version (PDF)
ChemComm Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/chemcomm Page 1 of 4 ChemComm Journal Name RSC Publishing COMMUNICATION Investigation of Oxidopyrylium-Alkene [5 + 2] Cycloaddition Conjugate Addition Cascade (C 3) Cite this: DOI: 10.1039/x0xx00000x Sequences Received 00th January 2012, Justin A. Simanis, C. Marshall Law, Erica L. Woodall, Christopher G. Hamaker, Accepted 00th January 2012 John R. Goodell, T. Andrew Mitchell* DOI: 10.1039/x0xx00000x www.rsc.org/ Novel oxidopyrylium-alkene [5 + 2] cycloaddition conjugate mechanistic information regarding the proposed reaction pathway addition cascade (C 3) sequences are described. Various (vide infra ). Pyranone-ketones 3b,c were subsequently converted to lactols 4b,c under basic conditions with aq. -

Steps Toward the Synthesis of 1,1'-Dideaza- Quinine: A
STEPS TOWARD THE SYNTHESIS OF 1,1’-DIDEAZA- QUININE: A PROOF OF CONCEPT by Nathan Schmitt Submitted in partial fulfillment of the requirements for Departmental Honors in the Department of Chemistry and Biochemistry Texas Christian University Fort Worth, Texas May 4, 2020 i STEPS TOWARD THE SYNTHESIS OF 1,1’-DIDEAZA- QUININE: A PROOF OF CONCEPT Project Approved: Supervising Professor: David Minter, Ph. D. Department of Chemistry and Biochemistry Jean-Luc Montchamp, Ph. D. Department of Chemistry and Biochemistry Greg Friedman, Ph. D. Department of Mathematics ii Abstract Quinine is a naturally occurring alkaloid with substantial medicinal relevance due to its historical role as an anti-malarial agent, although it is now used most often for special cases where the organism is resistant to newer drugs.1 While quinine is easily extracted from the bark of the Cinchona tree,2 the challenge of engineering a set of reactions to synthesize stereochemically pure quinine has captivated chemists for generations. Due to its four stereocenters, the synthesis of this molecule can yield up to sixteen different stereoisomers. The purpose of this study is to validate the conceptual route proposed by Stotter, Friedman, and Minter3 for the diastereoselective total synthesis of quinine via the preparation of racemic 1,1’-dideaza-quinine—a quinine analog lacking nitrogen atoms. While the total synthesis of quinine has been completed successfully by several other groups, our proposed route provides a novel process through a tandem, diastereoselective aldol addition and reduction to establish two of the four chiral centers in a single operation. This route avoids overly expensive reagents and provides a more concise synthetic scheme. -

Hemiacetal from Wikipedia, the Free Encyclopedia
Hemiacetal From Wikipedia, the free encyclopedia Hemiacetals and hemiketals are compounds that are derived from aldehydes and ketones respectively. The Greek word hèmi means half. These compounds are formed by formal addition of an alcohol to the carbonyl group. When the alcohol group is replaced by a second alkoxy group, an acetal or a ketal, respectively, is formed. Skeletal formula of a hemiacetal Contents 1 Formula and formation 1.1 Cyclic hemiacetals and hemiketals 2 Synthesis 3 Reactions 4 See also Hemiketal 5 References Formula and formation The general formula of a hemiacetal is R1R2C(OH)OR,[1] where R1 or R2 is often hydrogen and R (bonded to O) is not hydrogen. While in the IUPAC definition of a hemiacetal R1 or R2 may or may not be a hydrogen, in a hemiketal none of the Rgroups is an H. Hemiketals are regarded as hemiacetals that have no Rgroups being H, thus a subclass of the hemiacetals. [2] Formation of hemiacetals Above: 1ethoxybutan1ol, a hemiacetal. Below: 1,1diethoxybutan, an acetal Formation of hemiketals Cyclic hemiacetals and hemiketals Hemiacetals and hemiketals are generally unstable compounds. In some cases however, stable cyclic hemiacetals and hemiketals, called lactols,[3] can be readily formed, especially when 5 Fructopyranose and 6membered Ribopyranose rings are possible. In this case an Left a lactol of ribose, a cyclic hemiacetal. intramolecular OH Right a lactol of fructose, a cyclic hemiketal. group reacts with the carbonyl group. Glucose and many other aldoses exist as cyclic hemiacetals whereas fructose and similar ketoses exist as cyclic hemiketals.