Chapter 4. Functional Group Transformations: Oxidation and Reduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modern-Reduction-Methods.Pdf
Modern Reduction Methods Edited by Pher G. Andersson and Ian J. Munslow Related Titles Yamamoto, H., Ishihara, K. (eds.) Torii, S. Acid Catalysis in Modern Electroorganic Reduction Organic Synthesis Synthesis 2008 2006 ISBN: 978-3-527-31724-0 ISBN: 978-3-527-31539-0 Roberts, S. M. de Meijere, A., Diederich, F. (eds.) Catalysts for Fine Chemical Metal-Catalyzed Cross- Synthesis V 5 – Regio and Coupling Reactions Stereo-Controlled Oxidations 2004 and Reductions ISBN: 978-3-527-30518-6 2007 Online Book Wiley Interscience Bäckvall, J.-E. (ed.) ISBN: 978-0-470-09024-4 Modern Oxidation Methods 2004 de Vries, J. G., Elsevier, C. J. (eds.) ISBN: 978-3-527-30642-8 The Handbook of Homogeneous Hydrogenation 2007 ISBN: 978-3-527-31161-3 Modern Reduction Methods Edited by Pher G. Andersson and Ian J. Munslow The Editors All books published by Wiley-VCH are carefully produced. Nevertheless, authors, editors, and Prof. Dr. Pher G. Andersson publisher do not warrant the information Uppsala University contained in these books, including this book, to Department of Organic Chemistry be free of errors. Readers are advised to keep in Husargatan 3 mind that statements, data, illustrations, 751 23 Uppsala procedural details or other items may Sweden inadvertently be inaccurate. Dr. Ian J. Munslow Library of Congress Card No.: Uppsala University applied for Department of Biochemistry and Organic Chemistry Husargatan 3 British Library Cataloguing-in-Publication Data 751 23 Uppsala A catalogue record for this book is available from Sweden the British Library. Bibliographic information published by the Deutsche Nationalbibliothek Die Deutsche Nationalbibliothek lists this publication in the Deutsche Nationalbibliografi e; detailed bibliographic data are available on the Internet at <http://dnb.d-nb.de>. -

Lanthanide Replacement in Organic Synthesis: Calcium-Mediated Luche-Type Reduction of Α,Β-Functionalised Ketones
Lanthanide Replacement in Organic Synthesis: Calcium-mediated Luche-type Reduction of α,β-functionalised Ketones A thesis submitted by Nina Viktoria Forkel In partial fulfilment of the requirement for a degree of Doctor of Philosophy Imperial College London Department of Chemistry South Kensington Campus SW7 2AZ London United Kingdom September 2013 Lanthanide Replacement in Organic Synthesis: Calcium-mediated Luche-type Reduction of α,β-functionalised Ketones “Man kann sich die Weite und Möglichkeiten des Lebens gar nicht unerschöpflich genug denken.” (Rainer Maria Rilke) This thesis is dedicated to all my loved ones. Declaration of originality and statement of copyright Declaration of originality I, Nina V. Forkel, certify that the research described within this thesis was carried out in the Department of Chemistry at Imperial College London between October 2009 and September 2012. It was accomplished under the primary supervision of Dr Matthew J. Fuchter, Imperial College London, along with supervision from Dr David A. Henderson, Pfizer Ltd. The entire body of work is that of the author, unless otherwise stated to the contrary, and has not been submitted previously for a degree at this or any other university. Statement of copyright The copyright of this thesis rests with the author and is made available under a Creative Commons Attribution Non-Commercial No Derivatives licence. Researchers are free to copy, distribute or transmit the thesis on the condition that they attribute it, that they do not use it for commercial purposes, and that they do not alter, transform or build upon it. For any reuse or redistribution, researchers must make clear to others the licence terms of this work. -

Studies Toward Synthesis of Polycyclic Polyprenylated Acylphloroglucinols
University of Kentucky UKnowledge University of Kentucky Doctoral Dissertations Graduate School 2006 STUDIES TOWARD SYNTHESIS OF POLYCYCLIC POLYPRENYLATED ACYLPHLOROGLUCINOLS Roxana Ciochina University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Ciochina, Roxana, "STUDIES TOWARD SYNTHESIS OF POLYCYCLIC POLYPRENYLATED ACYLPHLOROGLUCINOLS" (2006). University of Kentucky Doctoral Dissertations. 291. https://uknowledge.uky.edu/gradschool_diss/291 This Dissertation is brought to you for free and open access by the Graduate School at UKnowledge. It has been accepted for inclusion in University of Kentucky Doctoral Dissertations by an authorized administrator of UKnowledge. For more information, please contact [email protected]. ABSTRACT OF DISSERTATION Roxana Ciochina The Graduate School University of Kentucky 2006 STUDIES TOWARD SYNTHESIS OF POLYCYCLIC POLYPRENYLATED ACYLPHLOROGLUCINOLS ABSTRACT OF DISSERTATION A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the College of Arts and Sciences at the University of Kentucky By Roxana Ciochina Lexington, KY Director: Dr. R. B. Grossman, Professor of Chemistry Lexington, KY 2006 ABSTRACT OF DISSERTATION STUDIES TOWARD SYNTHESIS OF POLYCYCLIC POLYPRENYLATED ACYLPHLOROGLUCINOLS Polycyclic polyprenylated acylphloroglucinols (PPAPs) are a class of compounds that reveal intriguing biological activities and interesting and challenging chemical structures. These products are claimed to possess antioxidant, antiviral, and antimitotic properties. Increasing interest is related to their function in the CNS as modulators of neurotransmitters associated to neuronal damaging and depression. All these features make PPAPs targets for synthesis. We decided to focus our own initial efforts in this area on the type A PPAP, nemorosone because we thought that its fairly simple structure relative to other PPAPs would present fewer hurdles as we developed our methodology. -

Oxidation of Secondary Alcohols to Ketones
Oxidation of secondary alcohols to ketones The oxidation of secondary alcohols to ketones is an important oxidation reaction in organic chemistry. Where a secondary alcohol is oxidised, it is converted to a ketone. The hydrogen from the hydroxyl group is lost along with the hydrogen bonded to the second carbon. The remaining oxygen then forms double bonds with the carbon. This leaves a ketone, as R1–COR2. Ketones cannot normally be oxidised any further because this would involve breaking a C–C bond, which requires too much energy.[1] The reaction can occur using a variety of oxidants. Contents Potassium dichromate PCC (Pyridinium chlorochromate) Dess–Martin oxidation Swern oxidation Oppenauer oxidation Fétizon oxidation See also References Potassium dichromate A secondary alcohol can be oxidised into a ketone using acidified potassium dichromate and heating under 2− 3+ reflux. The orange-red dichromate ion, Cr2O7 , is reduced to the green Cr ion. This reaction was once used in an alcohol breath test. PCC (Pyridinium chlorochromate) PCC, when used in an organic solvent, can be used to oxidise a secondary alcohol into a ketone. It has the advantage of doing so selectively without the tendency to over-oxidise. Dess–Martin oxidation The Dess–Martin periodinane is a mild oxidant for the conversion of alcohols to aldehydes or ketones.[2] The reaction is performed under standard conditions, at room temperature, most often in dichloromethane. The reaction takes between half an hour and two hours to complete. The product is then separated from the spent periodinane.[3] Swern oxidation Swern oxidation oxidises secondary alcohols into ketones using oxalyl chloride and dimethylsulfoxide. -

Alcohol Oxidation
Alcohol oxidation Alcohol oxidation is an important organic reaction. Primary alcohols (R-CH2-OH) can be oxidized either Mechanism of oxidation of primary alcohols to carboxylic acids via aldehydes and The indirect oxidation of aldehyde hydrates primary alcohols to carboxylic acids normally proceeds via the corresponding aldehyde, which is transformed via an aldehyde hydrate (R- CH(OH)2) by reaction with water. The oxidation of a primary alcohol at the aldehyde level is possible by performing the reaction in absence of water, so that no aldehyde hydrate can be formed. Contents Oxidation to aldehydes Oxidation to ketones Oxidation to carboxylic acids Diol oxidation References Oxidation to aldehydes Oxidation of alcohols to aldehydes is partial oxidation; aldehydes are further oxidized to carboxylic acids. Conditions required for making aldehydes are heat and distillation. In aldehyde formation, the temperature of the reaction should be kept above the boiling point of the aldehyde and below the boiling point of the alcohol. Reagents useful for the transformation of primary alcohols to aldehydes are normally also suitable for the oxidation of secondary alcohols to ketones. These include: Oxidation of alcohols to aldehydes and ketones Chromium-based reagents, such as Collins reagent (CrO3·Py2), PDC or PCC. Sulfonium species known as "activated DMSO" which can result from reaction of DMSO with electrophiles, such as oxalyl chloride (Swern oxidation), a carbodiimide (Pfitzner-Moffatt oxidation) or the complex SO3·Py (Parikh-Doering oxidation). Hypervalent iodine compounds, such as Dess-Martin periodinane or 2-Iodoxybenzoic acid. Catalytic TPAP in presence of excess of NMO (Ley oxidation). Catalytic TEMPO in presence of excess bleach (NaOCl) (Oxoammonium-catalyzed oxidation). -

Metabolic Carbonyl Reduction of Anthracyclines — Role in Cardiotoxicity and Cancer Resistance
Invest New Drugs DOI 10.1007/s10637-017-0443-2 REVIEW Metabolic carbonyl reduction of anthracyclines — role in cardiotoxicity and cancer resistance. Reducing enzymes as putative targets for novel cardioprotective and chemosensitizing agents Kamil Piska1 & Paulina Koczurkiewicz1 & Adam Bucki 2 & Katarzyna Wójcik-Pszczoła1 & Marcin Kołaczkowski2 & Elżbieta Pękala1 Received: 23 November 2016 /Accepted: 17 February 2017 # The Author(s) 2017. This article is published with open access at Springerlink.com Summary Anthracycline antibiotics (ANT), such as doxoru- monoHER, curcumin, (−)-epigallocatechin gallate, resvera- bicin or daunorubicin, are a class of anticancer drugs that are trol, berberine or pixantrone, and their modulating effect on widely used in oncology. Although highly effective in cancer the activity of ANT is characterized and discussed as potential therapy, their usefulness is greatly limited by their mechanism of action for novel therapeutics in cancer cardiotoxicity. Possible mechanisms of ANT cardiotoxicity treatment. include their conversion to secondary alcohol metabolites (i.e. doxorubicinol, daunorubicinol) catalyzed by carbonyl re- Keywords Anthracyclines . Cardiotoxicity . Resistance . ductases (CBR) and aldo-keto reductases (AKR). These me- Pharmacokinetics . Drug metabolism . Anticancer agents tabolites are suspected to be more cardiotoxic than their parent compounds. Moreover, overexpression of ANT-reducing en- zymes (CBR and AKR) are found in many ANT-resistant Introduction cancers. The secondary metabolites show decreased cytotoxic properties and are more susceptible to ABC-mediated efflux Anthracyclines (ANT) are a class of cell-cycle non-specific than their parent compounds; thus, metabolite formation is anticancer antibiotics that were first isolated from the considered one of the mechanisms of cancer resistance. Streptomyces genus in the early 1960s. -

Synthesis of Alkynyl Ribofuranosides
City University of New York (CUNY) CUNY Academic Works Dissertations and Theses City College of New York 2011 Synthesis of Alkynyl Ribofuranosides Christian Rodriguez CUNY City College How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/cc_etds_theses/25 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] SYNTHESIS OF ALKYNYL RIBOFURANOSIDES A Thesis Presented to The Faculty of the Chemistry Program The City College of New York In (Partial) Fulfillment of the Requirements for the Degree Master of Arts by Christian Rodriguez December, 2010 - 1 - Synthesis of Alkynyl Ribofuranosides By Christian Rodriguez Mentor: P. Meleties Table of Contents Chapter 1 1.1 Introduction 6 1.2 Preparation of ribonolactone template 8 1.3 Synthesis of protected ribonolactone 9 Chapter 2 2.1 Preparation of ethynyl ribofuranosides 10 2.2 Reaction with ethynylmagnesium bromide 12 2.3 Intramolecular cyclization of diyne diol 14 2.4 Reaction of ribonolactone with lithium acetylide 15 2.5 Boron trifluoride hemiacetal deoxygenation 15 2.6 Alternative deacetylation 16 Chapter 3 3.1 Selecting appropriate protecting group 19 3.2 Preparation of trimethylsilyl alkynyl 5-O-benzyl-2,3-O isopropylidene 19 ribofuranoside 3.3 Lewis acid promoted triethylsilane dehydroxylation mechanism 20 Chapter 4 4.1 Hemiacetal alkynylation 25 4.2 Hemiacetal nucleophilic addition mechanism 26 4.3 Intramolecular -
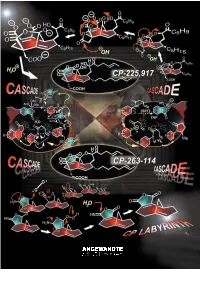
A Paradigm of How Endeavors in Total Synthesis Lead to Discoveries and Inventions in Organic Synthesis
REVIEWS The CP Molecule Labyrinth: A Paradigm of How Endeavors in Total Synthesis Lead to Discoveries and Inventions in Organic Synthesis K. C. Nicolaou* and Phil S. Baran Dedicated to Mrs. Niki Goulandris for her outstanding contributions to humanity and Planet Earth on the occasion of the opening of the GAIA Center for Environmental Research and Education at the Goulandris Natural History Museum in Athens, Greece. Imagine an artist carving a sculpture Herculean nature of the task and the ed Minotaur, which he accomplished from a marble slab and finding gold rewards that accompany it, one must through brilliance, skill, and bravery nuggets in the process. This thought is sense the details of the enterprise having traversed the famous labyrinth not a far-fetched description of the behind the scenes. A more vivid de- with the help of Ariadne. This story work of a synthetic chemist pursuing scription of total synthesis as a struggle from Greek mythology comes alive in the total synthesis of a natural product. against a tough opponent is perhaps modern synthetic expeditions toward At the end of the day, he or she will be appropriate to dramatize these ele- natural products as exemplified by the judged by the artistry of the final work ments of the experience. In this article total synthesis of the CP molecules and the weight of the gold discovered we describe one such endeavor of total which serve as a paradigm for modern in the process. However, as colorful as synthesis which, in addition to reaching total synthesis endeavors, where the this description of total synthesis may the target molecule, resulted in a objectives are discovery and invention be, it does not entirely capture the wealth of new synthetic strategies and in the broader sense of organic syn- essence of the endeavor, for there is technologies for chemical synthesis. -

20 More About Oxidation–Reduction Reactions
More About 20 Oxidation–Reduction Reactions OOC n important group of organic reactions consists of those that O A involve the transfer of electrons C from one molecule to another. Organic chemists H OH use these reactions—called oxidation–reduction reactions or redox reactions—to synthesize a large O variety of compounds. Redox reactions are also important C in biological systems because many of these reactions produce HH energy. You have seen a number of oxidation and reduction reactions in other chapters, but discussing them as a group will give you the opportunity to CH3OH compare them. In an oxidation–reduction reaction, one compound loses electrons and one com- pound gains electrons. The compound that loses electrons is oxidized, and the one that gains electrons is reduced. One way to remember the difference between oxidation and reduction is with the phrase “LEO the lion says GER”: Loss of Electrons is Oxi- dation; Gain of Electrons is Reduction. The following is an example of an oxidation–reduction reaction involving inorganic reagents: Cu+ + Fe3+ ¡ Cu2+ + Fe2+ In this reaction,Cu+ loses an electron, so Cu+ is oxidized. Fe3+ gains an electron, so Fe3+ is reduced. The reaction demonstrates two important points about oxidation– reduction reactions. First, oxidation is always coupled with reduction. In other words, a compound cannot gain electrons (be reduced) unless another compound in the reaction simultaneously loses electrons (is oxidized). Second, the compound that is oxidized (Cu+) is called the reducing agent because it loses the electrons that are used to reduce the other compound (Fe3+). Similarly, the compound that is reduced (Fe3+) is called the oxidizing agent because it gains the electrons given up by the other compound (Cu+) when it is oxidized. -

Asymmetric Total Synthesis of Congeners of Hydramycin, an Anthraquinone-Type Antitumor Agent
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2011 Asymmetric Total Synthesis of Congeners of Hydramycin, an Anthraquinone-Type Antitumor Agent Costyl Ngnouomeuchi Njiojob [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Heterocyclic Compounds Commons, Organic Chemicals Commons, and the Polycyclic Compounds Commons Recommended Citation Njiojob, Costyl Ngnouomeuchi, "Asymmetric Total Synthesis of Congeners of Hydramycin, an Anthraquinone-Type Antitumor Agent. " PhD diss., University of Tennessee, 2011. https://trace.tennessee.edu/utk_graddiss/1210 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Costyl Ngnouomeuchi Njiojob entitled "Asymmetric Total Synthesis of Congeners of Hydramycin, an Anthraquinone-Type Antitumor Agent." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Chemistry. David C. Baker, Major Professor We have read this dissertation and recommend its acceptance: Shawn Campagna, Ben Xue, Elizabeth Howell Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Asymmetric Total Synthesis of Congeners of Hydramycin, an Anthraquinone-Type Antitumor Agent A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville Costyl Ngnouomeuchi Njiojob December 2011 DEDICATION This dissertation is dedicated to my Parents Francois Ngnouomeuchi and Lydie T. -

Feni Nanoparticles with Carbon Armor As Sustainable Hydrogenation Catalysts: Towards Cite This: J
Journal of Materials Chemistry A View Article Online COMMUNICATION View Journal | View Issue FeNi nanoparticles with carbon armor as sustainable hydrogenation catalysts: towards Cite this: J. Mater. Chem. A,2014,2, 11591 biorefineries† Received 15th May 2014 Gianpaolo Chieffi, Cristina Giordano, Markus Antonietti and Davide Esposito* Accepted 28th May 2014 DOI: 10.1039/c4ta02457e www.rsc.org/MaterialsA Carbon supported FeNi nanoparticles were prepared by carbothermal and electronic properties of the active sites. Moreover, catalyst reduction of cellulose filter paper impregnated with Fe and Ni salts. stability is improved by inhibiting the sintering or the leaching The resulting carbon enwrapped alloy nanoparticles were employed of the active species through interaction with the second metal. as an efficient catalyst for the continuous hydrogenation of molecules The development of new sustainable hydrogenation catalysts Creative Commons Attribution 3.0 Unported Licence. obtainable from different fractions of lignocellulosic biomass. Scale- with superior stability is also highly desired for the successful up and time on stream tests over 80 hours proved the catalyst stable development of bioreneries for the conversion of raw ligno- and durable of over a wide range of conditions. cellulosic materials into chemicals and fuels, where poor selectivity and catalyst deactivation are sensibly affected by the high heterogeneity of biomass. In the present work we describe the sustainable synthesis of Introduction a carbonized lter paper (CFP) supported bimetallic FeNi alloy catalyst and investigate its activity during the continuous Catalytic hydrogenation has been one of the most studied hydrogenation of different functional groups and model This article is licensed under a reactions for decades. -

Oxidation States of Carbon Oxidation and Reduction in Biology
Oxidation States of Carbon • Addition of H (or “H-”) Reduction 2 • Loss of O2 or O H R H H carboxylic aldehyde alcohol alkane acid (or ketone) • Loss of H 2 Oxidation • Addition of O2 or O + Neither oxidation nor reduction: Addition or loss of H , H2O, or HX Oxidation and Reduction in Biology • Addition of H (or “H-”) Reduction 2 • Loss of O2 or O + h, in plants H OH carbon dioxide e.gg,g., glucose H O 4 C-O bonds per C HO 1 C-O bond per C 0 C-H bond per C HO OH 1 C-H bond per C H OH H H +O+ O2, in animals • Loss of H 2 Oxidation • Addition of O2 or O + Neither oxidation nor reduction: Addition or loss of H , H2O, or HX Biological Oxidation Gone Wrong component of glue alcohol 2 NAD+ dehydrogenase 2 NADH gamma-hydroxybutyrate (GHB, an intoxicant/ date rape drug) Dr. Kevin Carpenter, biochemical geneticist (specialist in pediatric metabolic disorders), Children’s Hospital at Westmead (Sydney), Australia. Behind him: The Agilent GC/MS New York Times, Nov. 8, 2007 used to analyze the Aqua Dots. “Sleuthing for Danger in Toy Beads” Hydrides as Reducing Agents Lithium aluminum hydride (LiAlH4) is a strong reducing agent. It will reduce any C=O containing functional group to an alcohol. + 2. H3O 1. LiAlH4 (or just H2O) carboxylic acid primary alcohol andthd then ano ther equivalent adds, unavoidably. One Reduced by LiAlH : equivalent of 4 H- adds, aldehyde ketone carboxylic ester acyl halide amide acid Hydrides as Reducing Agents Sodium borohydride (NaBH4) is a mild reducing agent.