Hemiacetal from Wikipedia, the Free Encyclopedia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Structures of Monosaccharides Hemiacetals
Structures of Monosaccharides Hemiacetals • Although, the open chain structures of monosaccharides are consistent with the chemistry of carbohydrates, in reality they are oversimplifications of the true structure of carbohydrates. • It is common knowledge that aldehydes react with alcohols to form hemiacetals. In cases where a molecule is a hydroxyaldehyde such as 4- hydroxybutanal or 5-hydroxypentanal, cyclic hemiacetals result. 9:47 AM 1 Structures of Monosaccharides Hemiacetals • Aldoses often contain an aldehyde group and several hydroxyl groups as part of the same molecule; they have a greater tendency of forming cyclic hemiacetals. In fact, in aqueous solution carbohydrates exist almost exclusively in the ring-closed form At equilibrium, the linear aldehyde or ketone structure represents less than 1% of the sugar present. • Five and six-membered rings are thermodynamically more stable than their corresponding four and seven membered rings, since they are less strained. • Five- (furanoses) and six-membered cyclic hemiacetals (pyranoses) are often more stable than their open-chain forms. In particular the six-membered rings which can adopt a chair conformation are 9:47 AM 2 essentially free from all types of strains. Structures of Monosaccharides Evidence for Existence of Monosacharides as Hemiacetals What physical, chemical and spectroscopic evidence support the existence of monosaccharide sugars as cyclic hemi-acetals. (a) Two anomers of glucose capable of existing independently with different physical (melting points and specific optical rotation) and chemical properties can be obtained by recrystallization. (b) the 1H-NMR and IR-spectra of solutions of pure sugars show the presence of mixtures (anomeric hemiacetals) and absence of an aldehydic peak is a sufficient indicator that the sugars exist in some other form other than the open-chain form. -

Studies Toward Synthesis of Polycyclic Polyprenylated Acylphloroglucinols
University of Kentucky UKnowledge University of Kentucky Doctoral Dissertations Graduate School 2006 STUDIES TOWARD SYNTHESIS OF POLYCYCLIC POLYPRENYLATED ACYLPHLOROGLUCINOLS Roxana Ciochina University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Ciochina, Roxana, "STUDIES TOWARD SYNTHESIS OF POLYCYCLIC POLYPRENYLATED ACYLPHLOROGLUCINOLS" (2006). University of Kentucky Doctoral Dissertations. 291. https://uknowledge.uky.edu/gradschool_diss/291 This Dissertation is brought to you for free and open access by the Graduate School at UKnowledge. It has been accepted for inclusion in University of Kentucky Doctoral Dissertations by an authorized administrator of UKnowledge. For more information, please contact [email protected]. ABSTRACT OF DISSERTATION Roxana Ciochina The Graduate School University of Kentucky 2006 STUDIES TOWARD SYNTHESIS OF POLYCYCLIC POLYPRENYLATED ACYLPHLOROGLUCINOLS ABSTRACT OF DISSERTATION A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the College of Arts and Sciences at the University of Kentucky By Roxana Ciochina Lexington, KY Director: Dr. R. B. Grossman, Professor of Chemistry Lexington, KY 2006 ABSTRACT OF DISSERTATION STUDIES TOWARD SYNTHESIS OF POLYCYCLIC POLYPRENYLATED ACYLPHLOROGLUCINOLS Polycyclic polyprenylated acylphloroglucinols (PPAPs) are a class of compounds that reveal intriguing biological activities and interesting and challenging chemical structures. These products are claimed to possess antioxidant, antiviral, and antimitotic properties. Increasing interest is related to their function in the CNS as modulators of neurotransmitters associated to neuronal damaging and depression. All these features make PPAPs targets for synthesis. We decided to focus our own initial efforts in this area on the type A PPAP, nemorosone because we thought that its fairly simple structure relative to other PPAPs would present fewer hurdles as we developed our methodology. -

Aldehydes Can React with Alcohols to Form Hemiacetals
340 14 . Nucleophilic substitution at C=O with loss of carbonyl oxygen You have, in fact, already met some reactions in which the carbonyl oxygen atom can be lost, but you probably didn’t notice at the time. The equilibrium between an aldehyde or ketone and its hydrate (p. 000) is one such reaction. O HO OH H2O + R1 R2 R1 R2 When the hydrate reverts to starting materials, either of its two oxygen atoms must leave: one OPh came from the water and one from the carbonyl group, so 50% of the time the oxygen atom that belonged to the carbonyl group will be lost. Usually, this is of no consequence, but it can be useful. O For example, in 1968 some chemists studying the reactions that take place inside mass spectrometers needed to label the carbonyl oxygen atom of this ketone with the isotope 18 O. 16 18 By stirring the ‘normal’ O compound with a large excess of isotopically labelled water, H 2 O, for a few hours in the presence of a drop of acid they were able to make the required labelled com- í In Chapter 13 we saw this way of pound. Without the acid catalyst, the exchange is very slow. Acid catalysis speeds the reaction up by making a reaction go faster by raising making the carbonyl group more electrophilic so that equilibrium is reached more quickly. The the energy of the starting material. We 18 also saw that the position of an equilibrium is controlled by mass action— O is in large excess. -

Educational Research Applications Abebe M, Et Al
Educational Research Applications Abebe M, et al. Educ Res Appl 5: 175. Review Article DOI: 10.29011/2575-7032.100175 Teaching Students Synthesizing Molecules Mimicking an Existing Drug against Covid-19 Moges Abebe1*, Lashan Eloise Knowles1, Bisrat Hailemeskel2 1Department of Biological and Physical Sciences, Saint Augustine University, Raleigh, NC, USA 2Department of Clinical & Administrative Pharmacy Sciences, College of Pharmacy, Howard University, NW Washington, DC, USA *Corresponding author: Moges Abebe, Department of Biological and Physical Sciences, Saint Augustine University, Raleigh, NC 27610, NC, USA Citation: Abebe M, Knowles LE, Hailemeskel B (2020) Teaching Students Synthesizing Molecules Mimicking an Existing Drug against Covid-19. Educ Res Appl 5: 175. DOI: 10.29011/2575-7032.100175 Received Date: 26 May 2020; Accepted Date: 01 June, 2020; Published Date: 06 June, 2020 Abstract End of semester organic chemistry course projects are valuable learning assessment tools while giving students a creative opportunity and sparking interest for further research investigations. The purpose of this year’s project was to teach students how to synthesize a molecule that potentially mimics an existing drug that works against the COVID-19. The available drugs chosen for the project are those that are proposed to work either by prohibiting the easy entry of the virus into respiratory tissues or those who deprive the virus’s ability to reproduce once they enter the cell. An investigative search in historical literature and the current conditions of the virus enabled students to create a unique and innovative product that requires a cumulative learned knowledge. History has shown that when a new virus becomes pandemic it takes time for researchers to create a drug, test the results, and gets approved by the Food and Drug Administration (FDA) for public availability. -

Synthesis of Alkynyl Ribofuranosides
City University of New York (CUNY) CUNY Academic Works Dissertations and Theses City College of New York 2011 Synthesis of Alkynyl Ribofuranosides Christian Rodriguez CUNY City College How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/cc_etds_theses/25 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] SYNTHESIS OF ALKYNYL RIBOFURANOSIDES A Thesis Presented to The Faculty of the Chemistry Program The City College of New York In (Partial) Fulfillment of the Requirements for the Degree Master of Arts by Christian Rodriguez December, 2010 - 1 - Synthesis of Alkynyl Ribofuranosides By Christian Rodriguez Mentor: P. Meleties Table of Contents Chapter 1 1.1 Introduction 6 1.2 Preparation of ribonolactone template 8 1.3 Synthesis of protected ribonolactone 9 Chapter 2 2.1 Preparation of ethynyl ribofuranosides 10 2.2 Reaction with ethynylmagnesium bromide 12 2.3 Intramolecular cyclization of diyne diol 14 2.4 Reaction of ribonolactone with lithium acetylide 15 2.5 Boron trifluoride hemiacetal deoxygenation 15 2.6 Alternative deacetylation 16 Chapter 3 3.1 Selecting appropriate protecting group 19 3.2 Preparation of trimethylsilyl alkynyl 5-O-benzyl-2,3-O isopropylidene 19 ribofuranoside 3.3 Lewis acid promoted triethylsilane dehydroxylation mechanism 20 Chapter 4 4.1 Hemiacetal alkynylation 25 4.2 Hemiacetal nucleophilic addition mechanism 26 4.3 Intramolecular -
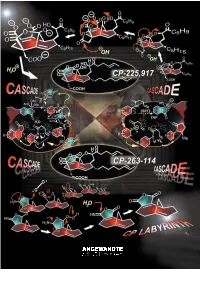
A Paradigm of How Endeavors in Total Synthesis Lead to Discoveries and Inventions in Organic Synthesis
REVIEWS The CP Molecule Labyrinth: A Paradigm of How Endeavors in Total Synthesis Lead to Discoveries and Inventions in Organic Synthesis K. C. Nicolaou* and Phil S. Baran Dedicated to Mrs. Niki Goulandris for her outstanding contributions to humanity and Planet Earth on the occasion of the opening of the GAIA Center for Environmental Research and Education at the Goulandris Natural History Museum in Athens, Greece. Imagine an artist carving a sculpture Herculean nature of the task and the ed Minotaur, which he accomplished from a marble slab and finding gold rewards that accompany it, one must through brilliance, skill, and bravery nuggets in the process. This thought is sense the details of the enterprise having traversed the famous labyrinth not a far-fetched description of the behind the scenes. A more vivid de- with the help of Ariadne. This story work of a synthetic chemist pursuing scription of total synthesis as a struggle from Greek mythology comes alive in the total synthesis of a natural product. against a tough opponent is perhaps modern synthetic expeditions toward At the end of the day, he or she will be appropriate to dramatize these ele- natural products as exemplified by the judged by the artistry of the final work ments of the experience. In this article total synthesis of the CP molecules and the weight of the gold discovered we describe one such endeavor of total which serve as a paradigm for modern in the process. However, as colorful as synthesis which, in addition to reaching total synthesis endeavors, where the this description of total synthesis may the target molecule, resulted in a objectives are discovery and invention be, it does not entirely capture the wealth of new synthetic strategies and in the broader sense of organic syn- essence of the endeavor, for there is technologies for chemical synthesis. -

Structures of Monosaccharides Hemiacetals
Disaccharides 10:51 AM 1 Disaccharides Definition • Disaccharides are carbohydrates consisting of two monosaccharide units linked via a glycosidic bond. Non-reducing disaccharide (1,1'-Glycosidic linkage) OH HO OH O HO O OH O OH OH HO OH HO O O HO OH + HO OH Glycosidic bond OH OH HO OH HO OH 6' 6 O O Reducing end 5' 1' 4 5 HO 4' O OH 3' 2' 3 2 1 HO OH HO OH Glycone Aglycone Reducing disaccharide (1,4'-Glycosidic linkage) • These disaccharides may be reducing or non-reducing sugars depending on the regiochemistry of the glycosidic 10:51 AM linkage between the two monosaccharides. 2 Nomenclature of Disaccharides • Since disaccharides are glycosides with two monosaccharide units linked through a glycosidic bond, their nomenclature requires the formulation of priority rules to identify which of the two monosaccharides of a disaccharide provides the parent name of the disaccharide and which one will be considered the substituent. • The nomenclature of disaccharides is based on the following considerations: i. Disaccharides with a free hemiacetal group (Reducing disaccharide) ii. Disaccharides without a free hemiacetal group (Non- Reducing Disaccharide) 10:51 AM 3 Nomenclature of Reducing Disaccharides • A disaccharide in which one glycosyl unit appears to have replaced the hydrogen atom of a hydroxyl group of the other is named as a glycosylglycose. The locants of the glycosidic linkage and the anomeric descriptor(s) must be given in the full name. • The parent sugar residue in such a reducing disaccharide is chosen on the basis of the following criteria: • The parent sugar residue is the one that includes the functional group most preferred by general principles of organic nomenclature. -

Chapter 16 ! Bonds with Hidden Leaving Groups (Sections 16.1-16.6 Excluding 16.3.1 and 16.5.3 ) O Formation and Reactivity of Acetals
O Chemistry 2600 Chapter 16 ! Bonds With Hidden Leaving Groups (sections 16.1-16.6 excluding 16.3.1 and 16.5.3 ) O Formation and Reactivity of Acetals • In Chapters 7 and 15 we saw how carbonyl compounds undergo addition reactions with nucleophiles. • We also saw how some of these reactions are reversible. • For example, recall the formation of a hydrate when an aldehyde or ketone reacts with water under acidic condition: 2 O Formation and Reactivity of Acetals • This reaction is reversible and after the loss of a leaving group, the carbonyl group is reformed: • Note that the oxygen that is lost could come from the initial carbonyl oxygen atom. • We say these molecules have a ‘hidden leaving group’. • This chapter explores the chemistry of the removal or replacement of these hidden leaving groups. 3 O Formation and Reactivity of Acetals • When an aldehyde/ketone reacts with an alcohol in the presence of acid, a hemiacetal is formed. • The only difference between hemiacetal formation and hydrate formation is the nucleophile (water vs alcohol). • Hemiacetals have a hidden leaving group and in the presence of alcohol and acid, react quickly to form an acetal. • Hemiacetal (tetrahedral carbon attached to –OH and –OR) • Acetal (tetrahedral carbon attached to two –OR groups) 4 O Formation and Reactivity of Acetals • Acetal formation: 5 O Formation and Reactivity of Acetals • Acetal formation is an equilibrium process, all the steps in the sequence can run in both directions. • When an acetal is converted to a carbonyl compound, the reaction is called hydrolysis because water is being used to break (lyse) the acetal. -

20H-Carbohydrates.Pdf
Carbohydrates Carbohydrates are compounds that have the general formula CnH2nOn Because CnH2nOn can also be written Cn(H2O)n, they appear to be “hydrates of carbon” Carbohydrates are also called “sugars” or “saccharides” Carbohydrates can be either aldoses (ald is for aldehyde and ose means a carbohydrate) or ketoses (ket is for ketone) OH OH O OH CH2OH CH2OH OHC HOH2C OH OH OH OH An Aldose A Ketose (D-Glucose) (D-Fructose) Carbohydrates Due to the multiple chiral centers along a linear carbon chain for carbohydrates, Emil Fischer developed the “Fischer Projection” in order to represent these compounds Remember how to draw a Fischer projection: 1) View the linear carbon chain along the vertical axis (always place the more oxidized carbon [aldehyde in an aldose] towards the top) 2) The horizontal lines are coming out of the page toward the viewer 3) Will need to change the viewpoint for each carbon so the horizontal substituents are always pointing towards the viewer CHO OH OH H OH HO H CH2OH = OHC H OH OH OH H OH CH2OH Emil Fischer (1852-1919) Carbohydrates The aldoses are thus all related by having an aldehyde group at one end, a primary alcohol group at the other end, and the two ends connected by a series of H-C-OH groups CHO CHO CHO CHO CHO H OH H OH H OH H OH HO H CH2OH H OH H OH H OH HO H CH2OH H OH H OH HO H CH2OH H OH HO H CH2OH CH2OH Aldotriose Aldotetrose Aldopentose Aldohexose Aldohexose D-glyceraldehyde D-erythose D-ribose D-allose L-allose The D-aldoses are named according to glyceraldehyde, the D refers to the configurational -

Application of Complex Aldol Reactions to the Total Synthesis of Phorboxazole B
J. Am. Chem. Soc. 2000, 122, 10033-10046 10033 Application of Complex Aldol Reactions to the Total Synthesis of Phorboxazole B David A. Evans,* Duke M. Fitch,1 Thomas E. Smith, and Victor J. Cee Contribution from the Department of Chemistry and Chemical Biology, HarVard UniVersity, Cambridge, Massachusetts 02138 ReceiVed June 29, 2000 Abstract: The synthesis of phorboxazole B has been accomplished in 27 linear steps and an overall yield of 12.6%. The absolute stereochemistry of the C4-C12,C33-C38, and C13-C19 fragments was established utilizing catalytic asymmetric aldol methodology, while the absolute stereochemistry of the C20-C32 fragment was derived from an auxiliary-based asymmetric aldol reaction. All remaining chirality was incorporated through internal asymmetric induction, with the exception of the C43 stereocenter which was derived from (R)-trityl glycidol. Key fragment couplings include a stereoselective double stereodifferentiating aldol reaction, a metalated oxazole alkylation, an oxazole-stabilized Wittig olefination, and a chelation-controlled addition of the fully elaborated alkenyl metal side chain. Introduction and HT29 (3.31 × 10-10 M), as well as leukemia CCRF-CBM (2.45 × 10-10 M), prostate cancer PC-3 (3.54 × 10-10 M), and Phorboxazoles A (1)andB(2) are marine natural products breast cancer MCF7 cell lines (5.62 × 10-10 M).2b In addition isolated from a recently discovered species of Indian Ocean to this impressive anticancer activity, phorboxazoles A and B sponge (genus Phorbas sp.) near Muiron Island, Western also exhibit potent in vitro antifungal activity against Candida Australia.2 These substances are representative of a new class albicans and Saccharomyces carlsbergensis at 0.1 µg/disk in of macrolides differing only in their C hydroxyl-bearing 13 the agar disk diffusion assay.2a stereocenters. -

Use of the Non-Aldol Aldol Process in the Synthesis of the C1–C11 Fragment of the Tedanolides: Use of Lactol Ethers in Place of Tetrahydrofurans
TETRAHEDRON LETTERS Pergamon Tetrahedron Letters 41 (2000) 9719–9723 Use of the non-aldol aldol process in the synthesis of the C1–C11 fragment of the tedanolides: use of lactol ethers in place of tetrahydrofurans Michael E. Jung* and Christopher P. Lee Department of Chemistry and Biochemistry, University of California, Los Angeles, CA 90095-1569, USA Received 15 August 2000; accepted 18 September 2000 Abstract The use of a lactol methyl ether 23 in place of the simple tetrahydrofuran 11 allows for the high yielding non-aldol aldol process to occur without concomitant tetrahydropyran formation (cf. 13) to give the desired product 24 in good yield. © 2000 Elsevier Science Ltd. All rights reserved. Keywords: non-aldol aldol; polypropionate synthesis; lactol methyl ethers. Tedanolide (1,R=OH) was isolated by Schmitz and co-workers in 1984 from the Caribbean 1 sponge Tedania ignis. The macrolide demonstrates its high cytotoxicity by displaying ED50’s of 250 pg/mL against human nasopharynx carcinoma and 16 pg/mL against in vitro lymphocytic leukemia. Seven years after tedanolide’s discovery, Fusetani and co-workers isolated 13-deoxy- tedanolide (2,R=H) from the Japanese sponge Mycale adhaerens.2 This macrolide is also extremely cytotoxic, exhibiting an IC50 of 94 pg/mL against P388 murine leukemia. Due to its powerful antitumor activity and complex structure, tedanolide has garnered considerable synthetic interest,3 including that of our group which uses the non-aldol aldol process.4 Disconnecting the tedanolide backbone retrosynthetically is quite straightforward, beginning with cleavage at the lactone moiety and at the C12C13 bond, which could be formed in the forward sense by an aldol reaction for 1 or an alkylation for 2, either prior to a macrolactoniza- tion or after simple ester formation. -

II Reduction Reactions
II Reduction Reactions Objectives By the end of this section you will: 1) be able to exploit the differences in reactivity of various reducing agents (hydride vs neutral reductants) in chemoselective reductions and be able to provide a mechanistic rationale to account for their differing reactivities; 2) be able to use the inherent chirality in a substrate to control the outcome of a reduction of proximal ketones to generate selectively syn and anti 1,3- and 1,2-diols; 3) be able to rationalise the outcome of these diastereoselective reactions using T.S. diagrams; 4) have gained an appreciation of the versatility of transition metals in reduction reactions; 5) have gained an appreciation of the synthetic utility of dissolving metal reductions; 6) be able to use radical chemistry for deoxygenation and reduction of halides. II.A Reduction of Carboxylic Acid Derivatives and Related Functionality OR' H ROH RO RO carboxylic acid aldehyde primary alcohol derivatives R N RNH2 RNO2 Issues of Reactivity and Selectivity Similar issues of selectivity and reactivity to those we encountered in the case of oxidation reactions also arise in reduction reactions. 1. Chemoselectivity. Many different functional groups can be reduced in a variety of ways. We often need to selectively reduce one functional group whilst leaving others intact (remember year 1 practical!). NaBH4 Sn, HCl OH O O O2N O2N H2N Chemoselective reductions from a practical in CHM1C3 2. In the case of carboxylic acid derivatives there are two possible reduction products: an aldehyde and an alcohol. Ideally we need methods for selectively accessing either product.