Chapter 3 Alcohols, Phenols, and Ethers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Working with Hazardous Chemicals
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices. In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red “Caution Notes” within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein. -

Cinnamoyl Esters of Lesquerella and Castor Oil: Novel Sunscreen Active Ingredients David L
Cinnamoyl Esters of Lesquerella and Castor Oil: Novel Sunscreen Active Ingredients David L. Compton*, Joseph A. Laszlo, and Terry A. Isbell New Crops and Processing Technology Research Unit, USDA, ARS, NCAUR, Peoria, Illinois 61604 ABSTRACT: Lesquerella and castor oils were esterified with querolin (2). Therefore, LO in essence contains two moles of cinnamic acid (CA) and 4-methoxycinnamic acid (MCA). Esteri- hydroxy functionality per mole of triglyceride (TG). fication of the hydroxy oils reached 85% completion with CA The hydroxy functionality of the FA of the TG can be ex- and 50% conversion with MCA. The hydroxy oils were esteri- ploited by esterification to form estolides. The majority of re- fied at 200°C under a nitrogen atmosphere within a sealed sys- search has focused on the estolides of hydroxy FA and TG tem. Unreacted CA and MCA were removed from the reaction formed with oleic acid. The esterification of hydroxy TG and mixtures by sublimation at 100°C under vacuum. The resultant free lesquerolic acid with oleic acid using a cobalt catalyst (4) methoxycinnamic oils possessed a broader, more blue-shifted or a lipase catalyst (5) has been reported. Also, the acid-cat- UV absorbance, 250 to 345 nm with a λmax of 305 nm, com- pared with the cinnamic oils, which absorbed from 260 to 315 alyzed formation of estolides from CO and LO with oleic acid has been patented (6). Recently, a detailed study of the effect nm, λmax of 270 nm. The methoxycinnamic oils provide better UV-B absorption and thus are better candidates to be used as of oleic acid concentration and temperature on catalyst-free sunscreen active ingredients. -

Structures of Monosaccharides Hemiacetals
Structures of Monosaccharides Hemiacetals • Although, the open chain structures of monosaccharides are consistent with the chemistry of carbohydrates, in reality they are oversimplifications of the true structure of carbohydrates. • It is common knowledge that aldehydes react with alcohols to form hemiacetals. In cases where a molecule is a hydroxyaldehyde such as 4- hydroxybutanal or 5-hydroxypentanal, cyclic hemiacetals result. 9:47 AM 1 Structures of Monosaccharides Hemiacetals • Aldoses often contain an aldehyde group and several hydroxyl groups as part of the same molecule; they have a greater tendency of forming cyclic hemiacetals. In fact, in aqueous solution carbohydrates exist almost exclusively in the ring-closed form At equilibrium, the linear aldehyde or ketone structure represents less than 1% of the sugar present. • Five and six-membered rings are thermodynamically more stable than their corresponding four and seven membered rings, since they are less strained. • Five- (furanoses) and six-membered cyclic hemiacetals (pyranoses) are often more stable than their open-chain forms. In particular the six-membered rings which can adopt a chair conformation are 9:47 AM 2 essentially free from all types of strains. Structures of Monosaccharides Evidence for Existence of Monosacharides as Hemiacetals What physical, chemical and spectroscopic evidence support the existence of monosaccharide sugars as cyclic hemi-acetals. (a) Two anomers of glucose capable of existing independently with different physical (melting points and specific optical rotation) and chemical properties can be obtained by recrystallization. (b) the 1H-NMR and IR-spectra of solutions of pure sugars show the presence of mixtures (anomeric hemiacetals) and absence of an aldehydic peak is a sufficient indicator that the sugars exist in some other form other than the open-chain form. -

1.1 10 Oxidation of Alcohols and Aldehydes
1.1 10 Oxidation of alcohols and aldehydes By the end of this spread, you should be able to … 1Describe the oxidation of primary alcohols to form aldehydes and carboxylic acids. 1Describe the oxidation of secondary alcohols to form ketones. 1Describe the oxidation of aldehydes to form carboxylic acids. Key definition Oxidation of alcohols You will recall from your AS chemistry studies that primary and secondary alcohols can A redox reaction is one in which both reduction and oxidation take place. be oxidised using an oxidising agent. s !SUITABLEOXIDISINGAGENTISASOLUTIONCONTAININGACIDIlEDDICHROMATEIONS + 2− H /Cr2O7 . s 4HEOXIDISINGMIXTURECANBEMADEFROMPOTASSIUMDICHROMATE +2Cr2O7, and sulfuric acid, H2SO. During the reaction, the acidified potassium dichromate changes from orange to green. Remember that tertiary alcohols are not oxidised by acidified dichromate ions. Primary alcohols Primary alcohols can be oxidised to aldehydes and to carboxylic acids (see AS Chemistry spread 2.2.3). H H H O H O Oxidation Oxidation H CCOH H CC H CC H H H H H OH Primary alcohol Aldehyde Carboxylic acid Figure 1 Ethanol oxidised to ethanal, and finally to ethanoic acid The equation for the oxidation of ethanol to the aldehyde ethanal is shown below. Note that the oxidising agent is shown as [O] – this simplifies the equation. CH3CH2OH + [O] }m CH3CHO + H2O When preparing aldehydes in the laboratory, you will need to distil the aldehyde from the reaction mixture as it is formed. This prevents the aldehyde from being oxidised further to a carboxylic acid. Key definition When making the carboxylic acid, the reaction mixture is usually heated under reflux before distilling the product off. -

Coast Guard, DHS Pt. 150, App. I
Coast Guard, DHS Pt. 150, App. I APPENDIX I TO PART 150—EXCEPTIONS Member of reactive group Compatible with TO THE CHART Propylene glycol (20) (a) The binary combinations listed below Oleum (0) ............................... Hexane (31) have been tested as prescribed in Appendix Dichloromethane (36) III and found not to be dangerously reactive. Perchloroethylene (36) These combinations are exceptions to the 1,2-Propylene glycol (20) ...... Diethylenetriamine (7) Compatibility Chart (Figure 1) and may be Polyethylene polyamines (7) Triethylenetetramine (7) stowed in adjacent tanks. Sodium dichromate, 70% (0) Methyl alcohol (20) Member of reactive group Compatible with Sodium hydrosulfide solution Methyl alcohol (20) (5). Acetone (18) .......................... Diethylenetriamine (7) Iso-Propyl alcohol (20) Acetone cyanohydrin (0) ....... Acetic acid (4) Sulfuric acid (2) ..................... Coconut oil (34) Acrylonitrile (15) ..................... Triethanolamine (8) Coconut oil acid (34) Palm oil (34) 1,3-Butylene glycol (20) ......... Morpholine (7) Tallow (34) 1,4-Butylene glycol (20) ......... Ethylamine (7) Sulfuric acid, 98% or less (2) Choice white grease tallow Triethanolamine (8) (34) gamma-Butyrolactone (0) ...... N-Methyl-2-pyrrolidone (9) Caustic potash, 50% or less Isobutyl alcohol (20) (b) The binary combinations listed below (5). Ethyl alcohol (20) have been determined to be dangerously re- Ethylene glycol (20) active, based on either data obtained in the Isopropyl alcohol (20) Methyl alcohol (20) literature or on laboratory testing which has iso-Octyl alcohol (20) been carried out in accordance with proce- Caustic soda, 50% or less (5) Butyl alcohol (20) dures prescribed in Appendix III. These com- tert-Butyl alcohol, Methanol binations are exceptions to the Compat- mixtures ibility Chart (Figure 1) and may not be Decyl alcohol (20) stowed in adjacent tanks. -

Unit One Part 2: Naming and Functional Groups
gjr-–- 1 Unit One Part 2: naming and functional groups • To write and interpret IUPAC names for small, simple molecules • Identify some common functional groups found in organic molecules O CH3 H3C N H N O N N O H3C S N O N H3C viagra™ (trade name) sildenafil (trivial name) 5-(2-ethoxy-5-(4-methylpiperazin-1-ylsulfonyl)phenyl)-1-methyl-3-propyl-1H- pyrazolo[4,3-d] pyrimidin-7(6H)-one dr gareth rowlands; [email protected]; science tower a4.12 http://www.massey.ac.nz/~gjrowlan gjr-–- 2 Systematic (IUPAC) naming PREFIX PARENT SUFFIX substituents / minor number of C principal functional functional groups AND multiple bond group index • Comprises of three main parts • Note: multiple bond index is always incorporated in parent section No. Carbons Root No. Carbons Root Multiple-bond 1 meth 6 hex Bond index 2 eth 7 hept C–C an(e) 3 prop 8 oct C=C en(e) 4 but 9 non C≡C yn(e) 5 pent 10 dec gjr-–- 3 Systematic (IUPAC) naming: functional groups Functional group Structure Suffix Prefix General form O –oic acid acid –carboxylic acid carboxy R-COOH R OH O O –oic anhydride anhydride –carboxylic anhydride R-C(O)OC(O)-R R O R O –oyl chloride acyl chloride -carbonyl chloride chlorocarbonyl R-COCl R Cl O –oate ester –carboxylate alkoxycarbonyl R-COOR R OR O –amide amide –carboxamide carbamoyl R-CONH2 R NH2 nitrile R N –nitrile cyano R-C≡N O –al aldehyde –carbaldehyde oxo R-CHO R H O ketone –one oxo R-CO-R R R alcohol R OH –ol hydroxy R-OH amine R NH2 –amine amino R-NH2 O ether R R –ether alkoxy R-O-R alkyl bromide bromo R-Br (alkyl halide) R Br (halo) (R-X) gjr-–- 4 Nomenclature rules 1. -

Phenol Health and Safety Guide
C - z_ IPCS INTERNATIONAL._ PROGRAMME ON CHEMICAL SAFETY OD 341 Health and Safety Guide No. 88 .P5 94 Ph c.2 PHENOL HEALTH AND SAFETY GUIDE ' UNITED NATIONS INTERNATIONAL ENVffiONMENTPROG~E LABOUR ORGANISATION WORLD HEALTH ORGANIZATION WORLD HEALTH ORGANIZATION, GENEVA 1994 1 I) 1\ ' ~Ii>cs Other HEALTH AND SAFETY GUIDES available: (continued on inside back cover) Acrolein (No . 67, 1992) Endrin (No. 60, 1991) Acrylamide (No. 45 , 1991) Epichlorohydrin (No. 8, 1987) Acrylonitrile (No. I, 1986) Ethylene oxide (No. 16, 1988) Aldicarb (No. 64, 1991) Fenitrothion (No. 65, 1991) Aldrin and dieldrin (No. 21 , 1988) Fenvalerate (No. 34, 1989) Allethrins (No. 24, 1989) Folpet (No. 72, 1992) Amitrole (No. 85 , 1994) Formaldehyde (No. 57, 1991) Ammonia (No. 37, 1990) Heptachlor (No. 14, 1988) Arsenic compounds, inorganic, other than Hexachlorobutadiene (No. 84, 1993) arsine (No. 70, 1992) Hexachlorocyclohexanes, alpha- and Atrazine (No. 47, 1990) beta- (No. 53, 1991) Barium (No. 46 , 1991) Hexachlorocyclopentadiene (No. 63 , 1991) Benomyl (No. 81, 1993) n-Hexane (No. 59, 1991) Bentazone (No. 48, 1990) Hydrazine (No. 56, 1991) Beryllium (No. 44, 1990) Isobenzan (No. 61 , 1991) !-Butanol (No. 3, 1987) Isobutanol (No. 9, 1987) 2-Butanol (No. 4, 1987) Kelevan (No. 2, 1987) ten-Butanol (No. 7, 1987) Lindane (No. 54 , 1991) Camphechlor (No. 40, 1990) Magnetic fields (No. 27, 1990) Captafol (No. 49, 1990) Methamidophos (No. 79, 1993) Captan (No. 50, 1990) Methyl bromide (Bromomethane) (No. 86, 1994) Carbaryl (No. 78, 1993) Methyl isobutyl ketone (No. 58, 1991) Carbendazim (No. 82, 1993) Methyl parathion (No. 75, 1992) Chlordane (No. 13 , 1988) Methylene chloride (No. -
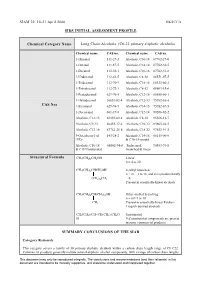
Long Chain Alcohols (C6-22 Primary Aliphatic Alcohols)
SIAM 22, 18-21 April 2006 UK/ICCA SIDS INITIAL ASSESSMENT PROFILE Chemical Category Name Long Chain Alcohols (C6-22 primary aliphatic alcohols) Chemical name CAS no. Chemical name CAS no. 1-Hexanol 111-27-3 Alcohols, C16-18 67762-27-0 1-Octanol 111-87-5 Alcohols, C14-18 67762-30-5 1-Decanol 112-30-1 Alcohols, C10-16 67762-41-8 1-Undecanol 112-42-5 Alcohols, C8-18 68551-07-5 1-Tridecanol 112-70-9 Alcohols, C14-16 68333-80-2 1-Tetradecanol 112-72-1 Alcohols, C6-12 68603-15-6 1-Pentadecanol 629-76-5 Alcohols, C12-16 68855-56-1 1-Hexadecanol 36653-82-4 Alcohols, C12-13 75782-86-4 CAS Nos 1-Eicosanol 629-96-9 Alcohols, C14-15 75782-87-5 1-Docosanol 661-19-8 Alcohols, C12-14 80206-82-2 Alcohols, C12-15 63393-82-8 Alcohols, C8-10 85566-12-7 Alcohols, C9-11 66455-17-2 Alcohols, C10-12 85665-26-5 Alcohols, C12-18 67762-25-8 Alcohols, C18-22 97552-91-5 9-Octadecen-1-ol 143-28-2 Alcohols, C14-18. 68155-00-0 (9Z)- & C16-18-unsatd Alcohols, C16-18 68002-94-8 Tridecanol, 90583-91-8 & C18 Unsaturated branched & linear Structural Formula CH3(CH2)nCH2OH Linear n = 4 to 20 CH3(CH2)nCHCH2OH 2-Alkyl branched n + m = 3 to 18, and m is predominantly (CH2)mCH3 = 0. Present in essentially-linear alcohols CH3(CH2)nCH(CH2)mOH Other-methyl branching n + m= 9 or 10 CH3 Present in essentially-linear Fischer- Tropsch derived alcohols CH3(CH2)7CH=CH(CH2)7CH2O Unsaturated H 9-Z unsaturated components are present in some commercial products. -

A Critical Study on Chemistry and Distribution of Phenolic Compounds in Plants, and Their Role in Human Health
IOSR Journal of Environmental Science, Toxicology and Food Technology (IOSR-JESTFT) e-ISSN: 2319-2402,p- ISSN: 2319-2399. Volume. 1 Issue. 3, PP 57-60 www.iosrjournals.org A Critical Study on Chemistry and Distribution of Phenolic Compounds in Plants, and Their Role in Human Health Nisreen Husain1, Sunita Gupta2 1 (Department of Zoology, Govt. Dr. W.W. Patankar Girls’ PG. College, Durg (C.G.) 491001,India) email - [email protected] 2 (Department of Chemistry, Govt. Dr. W.W. Patankar Girls’ PG. College, Durg (C.G.) 491001,India) email - [email protected] Abstract: Phytochemicals are the secondary metabolites synthesized in different parts of the plants. They have the remarkable ability to influence various body processes and functions. So they are taken in the form of food supplements, tonics, dietary plants and medicines. Such natural products of the plants attribute to their therapeutic and medicinal values. Phenolic compounds are the most important group of bioactive constituents of the medicinal plants and human diet. Some of the important ones are simple phenols, phenolic acids, flavonoids and phenyl-propanoids. They act as antioxidants and free radical scavengers, and hence function to decrease oxidative stress and their harmful effects. Thus, phenols help in prevention and control of many dreadful diseases and early ageing. Phenols are also responsible for anti-inflammatory, anti-biotic and anti- septic properties. The unique molecular structure of these phytochemicals, with specific position of hydroxyl groups, owes to their powerful bioactivities. The present work reviews the critical study on the chemistry, distribution and role of some phenolic compounds in promoting health-benefits. -

Physicochemical Properties of Organic Medicinal Agents
Principles of Drug Action 1, Spring 2005, Esters ESTERS AND RELATED CARBOXYLIC ACID DERIVATIVES Jack DeRuiter I. Structure and Preparation Esters are derivatives of carboxylic acids that arise via replacement of the hydroxyl (OH) portion of the acid COOH function with an "ether" moiety (-OR): O O H C C O C O Acid Ester Note that replacement of the acid OH group with an "ether" moiety removes the acidic function from the parent structure (acid) resulting in the formation of non-acidic (neutral, but somewhat polar) compounds (esters). Esters can be sub-classified based on their general structure as aliphatic, aromatic or cyclic (called "lactones") as illustrated by the examples below: O O CH2CH3 CH2CH3 O CH3 O O O Aliphatic Ester Aromatic Ester Cyclic Ester (Lactone) A variety of methods have been developed for the preparation of esters. Most of these methods involve reaction of an alcohol with an "activated carboxylic acid" compound (i.e. acid chloride): O O H C C X OC C O X- Ester "Activated" acid (X=Cl) Alcohol (Electrophile) (Nucleophile) The ester functionality does not introduce a center of asymmetry and thus optical and geometric isomerism does not result from the presence of this functional group. The ester functionality (the carbonyl and ether oxygen) is composed of an sp2 hybridized carbon so it cannot be chiral, and since there is free rotation about the ether bond geometric isomerism also is not possible at the sp2 center. 1 Principles of Drug Action 1, Spring 2005, Esters II. Solubility of Esters Esters contain carbonyl (C=O) and ether (O-C) dipoles arising from covalent bonding between electronegative oxygen atoms and electronically neutral carbon atoms. -
![United States Patent [191 [11] Patent Number: 5,070,175 Tsumura Et Al](https://docslib.b-cdn.net/cover/3510/united-states-patent-191-11-patent-number-5-070-175-tsumura-et-al-583510.webp)
United States Patent [191 [11] Patent Number: 5,070,175 Tsumura Et Al
United States Patent [191 [11] Patent Number: 5,070,175 Tsumura et al. [45] Date of Patent: Dec. 3, 1991 [54] METHOD FOR THE PREPARATION OF AN Primary Examiner-Morton Foelak ORGANOPOLYSILOXANE CONTAINING Attorney, Agent, or Firm-Millen, White & Zelano TETRAFUNCI'IONAL SILOXANE UNITS [57] ABSTRACT [75] Inventors: Hiroshi Tsumura; Kiyoyuki Mutoh, An ef?cient and economically advantageous method is both of Gunma; Kazushi Satoh, proposed for the preparation of an organopolysiloxane Tokyo; Ken-ichi Isobe, Gunma, all of comprising tetrafunctional siloxane units, i.e. Q units, Japan and, typically, monofunctional siloxy units, i.e. M units, [73] Assignee: Shin-Etsu Chemical Co., Ltd., Tokyo, and useful as a reinforcing agent in silicone rubbers. The Japan method comprises the steps of: mixing the reactants for providing the Q and M units, such as ethyl orthosilicate [21] Appl. No.;. 706,148 and trimethyl methoxy silane, in a desired molar ratio; [22] Filed: May 28, 1991 and heating the mixture at a temperature higher by at least 10° C. than the boiling point of the mixture under [30] Foreign Application Priority Data normal pressure in a closed vessel in the presence of May 29, 1990 [JP] Japan ............ .Q .................. .. 2-l39ll9 water and a catalyst such as a sulfonic acid group-con taining compound. In addition to the greatly shortened [51] Int. 01.5 ............................................ .. C08G 77/06 reaction time and remarkably decreased contents of [52] U.S. c1. ...................................... .. 528/12; 528/10; residual alkoxy groups and gelled matter in the product, 528/21; 528/23; 528/34; 528/36 the method is advantageous also in respect of the ab [58] Field of Search .................... -

Halogenated Ether, Alcohol, and Alkane Anesthetics Activate TASK-3 Tandem Pore Potassium Channels Likely Through a Common Mechanism S
Supplemental material to this article can be found at: http://molpharm.aspetjournals.org/content/suppl/2017/03/21/mol.117.108290.DC1 1521-0111/91/6/620–629$25.00 https://doi.org/10.1124/mol.117.108290 MOLECULAR PHARMACOLOGY Mol Pharmacol 91:620–629, June 2017 Copyright ª 2017 by The American Society for Pharmacology and Experimental Therapeutics Halogenated Ether, Alcohol, and Alkane Anesthetics Activate TASK-3 Tandem Pore Potassium Channels Likely through a Common Mechanism s Anita Luethy, James D. Boghosian, Rithu Srikantha, and Joseph F. Cotten Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts (A.L., J.D.B., and J.F.C.); Department of Anesthesia, Kantonsspital Aarau, Aarau, Switzerland (A.L.); Carver College of Medicine, University of Iowa, Iowa City, Iowa (R.S.) Received January 7, 2017; accepted March 20, 2017 Downloaded from ABSTRACT The TWIK-related acid-sensitive potassium channel 3 (TASK-3; hydrate (165% [161–176]) . 2,2-dichloro- . 2-chloro 2,2,2- KCNK9) tandem pore potassium channel function is activated by trifluoroethanol . ethanol. Similarly, carbon tetrabromide (296% halogenated anesthetics through binding at a putative anesthetic- [245–346]), carbon tetrachloride (180% [163–196]), and 1,1,1,3,3,3- binding cavity. To understand the pharmacologic requirements for hexafluoropropanol (200% [194–206]) activate TASK-3, whereas molpharm.aspetjournals.org TASK-3 activation, we studied the concentration–response of the larger carbon tetraiodide and a-chloralose inhibit. Clinical TASK-3 to several anesthetics (isoflurane, desflurane, sevoflurane, agents activate TASK-3 with the following rank order efficacy: halothane, a-chloralose, 2,2,2-trichloroethanol [TCE], and chloral halothane (207% [202–212]) .