Chemistry and Physics of Lipids Effects of Ether Vs. Ester Linkage On
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Physicochemical Properties of Organic Medicinal Agents
Principles of Drug Action 1, Spring 2005, Esters ESTERS AND RELATED CARBOXYLIC ACID DERIVATIVES Jack DeRuiter I. Structure and Preparation Esters are derivatives of carboxylic acids that arise via replacement of the hydroxyl (OH) portion of the acid COOH function with an "ether" moiety (-OR): O O H C C O C O Acid Ester Note that replacement of the acid OH group with an "ether" moiety removes the acidic function from the parent structure (acid) resulting in the formation of non-acidic (neutral, but somewhat polar) compounds (esters). Esters can be sub-classified based on their general structure as aliphatic, aromatic or cyclic (called "lactones") as illustrated by the examples below: O O CH2CH3 CH2CH3 O CH3 O O O Aliphatic Ester Aromatic Ester Cyclic Ester (Lactone) A variety of methods have been developed for the preparation of esters. Most of these methods involve reaction of an alcohol with an "activated carboxylic acid" compound (i.e. acid chloride): O O H C C X OC C O X- Ester "Activated" acid (X=Cl) Alcohol (Electrophile) (Nucleophile) The ester functionality does not introduce a center of asymmetry and thus optical and geometric isomerism does not result from the presence of this functional group. The ester functionality (the carbonyl and ether oxygen) is composed of an sp2 hybridized carbon so it cannot be chiral, and since there is free rotation about the ether bond geometric isomerism also is not possible at the sp2 center. 1 Principles of Drug Action 1, Spring 2005, Esters II. Solubility of Esters Esters contain carbonyl (C=O) and ether (O-C) dipoles arising from covalent bonding between electronegative oxygen atoms and electronically neutral carbon atoms. -

Halogenated Ether, Alcohol, and Alkane Anesthetics Activate TASK-3 Tandem Pore Potassium Channels Likely Through a Common Mechanism S
Supplemental material to this article can be found at: http://molpharm.aspetjournals.org/content/suppl/2017/03/21/mol.117.108290.DC1 1521-0111/91/6/620–629$25.00 https://doi.org/10.1124/mol.117.108290 MOLECULAR PHARMACOLOGY Mol Pharmacol 91:620–629, June 2017 Copyright ª 2017 by The American Society for Pharmacology and Experimental Therapeutics Halogenated Ether, Alcohol, and Alkane Anesthetics Activate TASK-3 Tandem Pore Potassium Channels Likely through a Common Mechanism s Anita Luethy, James D. Boghosian, Rithu Srikantha, and Joseph F. Cotten Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts (A.L., J.D.B., and J.F.C.); Department of Anesthesia, Kantonsspital Aarau, Aarau, Switzerland (A.L.); Carver College of Medicine, University of Iowa, Iowa City, Iowa (R.S.) Received January 7, 2017; accepted March 20, 2017 Downloaded from ABSTRACT The TWIK-related acid-sensitive potassium channel 3 (TASK-3; hydrate (165% [161–176]) . 2,2-dichloro- . 2-chloro 2,2,2- KCNK9) tandem pore potassium channel function is activated by trifluoroethanol . ethanol. Similarly, carbon tetrabromide (296% halogenated anesthetics through binding at a putative anesthetic- [245–346]), carbon tetrachloride (180% [163–196]), and 1,1,1,3,3,3- binding cavity. To understand the pharmacologic requirements for hexafluoropropanol (200% [194–206]) activate TASK-3, whereas molpharm.aspetjournals.org TASK-3 activation, we studied the concentration–response of the larger carbon tetraiodide and a-chloralose inhibit. Clinical TASK-3 to several anesthetics (isoflurane, desflurane, sevoflurane, agents activate TASK-3 with the following rank order efficacy: halothane, a-chloralose, 2,2,2-trichloroethanol [TCE], and chloral halothane (207% [202–212]) . -

BENZENE AS a LARVICIDE for SCREW WORMS1 the Larval Stage
BENZENE AS A LARVICIDE FOR SCREW WORMS1 By D. C. PARMAN Assistant Entomologist, Investigations of Insects Affecting the Health of Animals, Bureau of Entomology, United States Department of Agriculture INTRODUCTION The larval stage of CocMiomyia macellaria Fab., generally known amonff stock raisers in the Southwest as the screw worm, causes con- siderable loss to the livestock industry, estimated as high as $5,000,000 in some years. It has been apparent that the larvicides used to kill the worms are either toxic to the animal or at least in most cases detrimental to the healing of the wounds. This toxicity was at first attributed to the screw worm, but as many cases were ob- served where the animal was practically consumed by the larvae and still lived until the loss of olood or injury to some vital organ brought de^th, it was surmised that the treatments with larvicides were the cause of many deaths. During the summer of 1916 syste- matic work was begun to find a more efficient larvicide than the phenols and chloroform which were generally used. At first an attempt was made to add something to these larvicides to counteract the toxic properties. As this was not successful it was deemed best to look for a chemical that might be used with more satis- factory results. Several chemical groups were studied for possible larvicides. EXPERIMENTAL PROCEDURE All available chemicals with possible larvicidal value were selected for laboratory tests to determine whether they would kill the larvae of the screw-worm fly. The first tests were made by pouring the chemical on a number of larvae in a tube, or dusting on just enough to cover the larvae. -

Discovery of Inhibitors for the Ether Lipid-Generating Enzyme AGPS As Anti-Cancer Agents † # ‡ # § # † § Valentina Piano, , Daniel I
Articles pubs.acs.org/acschemicalbiology Discovery of Inhibitors for the Ether Lipid-Generating Enzyme AGPS as Anti-Cancer Agents † # ‡ # § # † § Valentina Piano, , Daniel I. Benjamin, , Sergio Valente, , Simone Nenci, Biagina Marrocco, § ∥ ⊥ ‡ † Antonello Mai, , Alessandro Aliverti, Daniel K. Nomura,*, and Andrea Mattevi*, † Department of Biology and Biotechnology, University of Pavia, via Ferrata 9, 27100 Pavia, Italy ‡ Program in Metabolic Biology, Department of Nutritional Sciences and Toxicology, University of California, Berkeley, Berkeley, California 94720, United States § Department of Drug Chemistry and Technologies, University “La Sapienza”, P. le A. Moro 5, Roma 00185, Italy ∥ Pasteur Institute, Cenci Bolognetti Foundation, P.le A. Moro 5, Roma 00185, Italy ⊥ Department of Biosciences, University of Milano, Via Festa del Perdono, 7, 20122 Milano, Italy *S Supporting Information ABSTRACT: Dysregulated ether lipid metabolism is an important hallmark of cancer cells. Previous studies have reported that lowering ether lipid levels by genetic ablation of the ether lipid-generating enzyme alkyl-glycerone phosphate synthase (AGPS) lowers key structural and oncogenic ether lipid levels and alters fatty acid, glycerophospholipid, and eicosanoid metabolism to impair cancer pathogenicity, indicating that AGPS may be a potential therapeutic target for cancer. In this study, we have performed a small-molecule screen to identify candidate AGPS inhibitors. We have identified several lead AGPS inhibitors and have structurally characterized their interactions with the enzyme and show that these inhibitors bind to distinct portions of the active site. We further show that the lead AGPS inhibitor 1a selectively lowers ether lipid levels in several types of human cancer cells and impairs their cellular survival and migration. We provide here the first report of in situ-active pharmacological tools for inhibiting AGPS, which may provide chemical scaffolds for future AGPS inhibitor development for cancer therapy. -
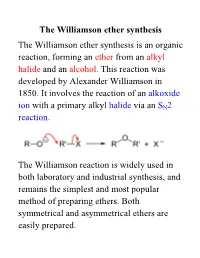
Williamson Ether Synthesis the Williamson Ether Synthesis Is an Organic Reaction, Forming an Ether from an Alkyl Halide and an Alcohol
The Williamson ether synthesis The Williamson ether synthesis is an organic reaction, forming an ether from an alkyl halide and an alcohol. This reaction was developed by Alexander Williamson in 1850. It involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. The Williamson reaction is widely used in both laboratory and industrial synthesis, and remains the simplest and most popular method of preparing ethers. Both symmetrical and asymmetrical ethers are easily prepared. The reaction for this week: an example of a Williamson ether synthesis acetaminophen ethyl iodide phenacetin starting material reagent product Phenacetin may be synthesized as an example of the Williamson ether synthesis The first synthesis of phenacetin was reported in 1878 by Harmon Morse. Procedure 1. Weigh an Extra-Strength Tylenol tablet. Pulverize the tablet with mortar and pestle. Weigh out 0.22 g and place it in a dry 15-ml round-bottom flask along with 0.28 g of finely pulverized K2CO3 (mortar and pestle) and 3.0 mL of butanone. Carefully add 0.28 mL of ethyl iodide with a syringe. 2. Add a stir bar; attach a microscale water-cooled condenser to the flask. Heat the mixture under reflux directly on a hot plate at medium setting for 1 hour. In the meantime, obtain the IR of acetaminophen. 3. Turn off the heat. Allow the mixture to cool down. Add 4 mL of water to the flask and transfer its contents to a 16 x 125 mm test tube with a screw cap. Rinse round-bottom flask 4 times with 1 mL of tert-butyl methyl ether (BME) and add the rinsings to the test tube. -

Reassessment of 3 Tolerance Exemptions for Ethylene Glycol
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY - +,TE* sr4, WASHINGTON, D.C. 20460 Q c, OFFICE OF PREVENTION, PESTICIDES, AND TOXIC SUBSTANCES DATE: June 29,2006 ACTION MEMORANDUM SUBJECT: Reassessment of 3 Tolerance Exemptions for Ethylene Glycol, Diethylene Glycol, and the Combination of Diethylene Glycol Monomethyl Ether, Diethylene Glycol Monoethyl Ether, and Diethylene Glycol Monobutyl Ether FROM: Pauline Wagner, Chief F b.~!!<Lo 'v \ 3~~10 b Inert Ingredient Assessment Branch Registration Division (7505P) TO: Lois A. Rossi, Director Registration Division (7505P) 1. FQPA REASSESSMENT ACTION Action: Reassessment of three inert exemptions from the requirement of a tolerance. The reassessment decision is to maintain the inert tolerance exemptions "as-is." Table 1. Tolerance Exemptions Being Reassessed in this Document CM~fl,~aa,it Appeara in the CFR CAS iT01muw Registry Number @.I@,- Bxemption $in$@ Uses Name %SOa ,. Exprmsion. Antifreeze, deactivator for all pesticides 107-21-1 920 Ethylene glycol - - - used before crop emerges from soil and in 1,2-Ethanediol herbicides before or after crop emerges Deactivator, adjuvant for formulations used before crop emerges from soil and 11 1-46-6 920 Diethylene glycol --- deactivator for formulations used before Ethanol, 2,2'-oxybis- (9CI) crop emerges from soil, stabilizer Diethylene glycol 1 11-77-3 monomethyl ether Ethanol, 2-(2-methoxyethoxy)- 920 Diethylene glycol monoethyl - - - Deactivator for formulations used before 1 1 1-90-0 ether crop emerges from soil, stabilizer Ethanol, 2-(2-ethoxyethoxy)- Diethylene glycol monobutyl 112-34-5 ether Ethanol, 2-(2-butoxyethoxy)- a. Residues listed in 40 CFR 180.920 are exempted from the requirement of a tolerance when used in accordance with good agricultural practice as inert (or occasionally active) ingredients in pesticide formulations applied to growing crops only. -

Reactions of Alkenes and Alkynes
05 Reactions of Alkenes and Alkynes Polyethylene is the most widely used plastic, making up items such as packing foam, plastic bottles, and plastic utensils (top: © Jon Larson/iStockphoto; middle: GNL Media/Digital Vision/Getty Images, Inc.; bottom: © Lakhesis/iStockphoto). Inset: A model of ethylene. KEY QUESTIONS 5.1 What Are the Characteristic Reactions of Alkenes? 5.8 How Can Alkynes Be Reduced to Alkenes and 5.2 What Is a Reaction Mechanism? Alkanes? 5.3 What Are the Mechanisms of Electrophilic Additions HOW TO to Alkenes? 5.1 How to Draw Mechanisms 5.4 What Are Carbocation Rearrangements? 5.5 What Is Hydroboration–Oxidation of an Alkene? CHEMICAL CONNECTIONS 5.6 How Can an Alkene Be Reduced to an Alkane? 5A Catalytic Cracking and the Importance of Alkenes 5.7 How Can an Acetylide Anion Be Used to Create a New Carbon–Carbon Bond? IN THIS CHAPTER, we begin our systematic study of organic reactions and their mecha- nisms. Reaction mechanisms are step-by-step descriptions of how reactions proceed and are one of the most important unifying concepts in organic chemistry. We use the reactions of alkenes as the vehicle to introduce this concept. 129 130 CHAPTER 5 Reactions of Alkenes and Alkynes 5.1 What Are the Characteristic Reactions of Alkenes? The most characteristic reaction of alkenes is addition to the carbon–carbon double bond in such a way that the pi bond is broken and, in its place, sigma bonds are formed to two new atoms or groups of atoms. Several examples of reactions at the carbon–carbon double bond are shown in Table 5.1, along with the descriptive name(s) associated with each. -

In This Handout, All of Our Functional Groups Are Presented As Condensed Line Formulas, 2D and 3D Formulas and with Nomenclature Prefixes and Suffixes (If Present)
In this handout, all of our functional groups are presented as condensed line formulas, 2D and 3D formulas and with nomenclature prefixes and suffixes (if present). Organic names are built on a foundation of alkanes, alkenes and alkynes. Those examples are presented first and you need to know those rules. The strategies can be found in Chapter 4 of our textbook (alkanes: pages 93-98, cycloalkanes 102-104, alkenes: pages 104-110, alkynes: pages 112-113 and combinations of all of them 113-115). After introducing examples of alkanes, alkenes, alkynes and combinations of them, the functional groups are presented in order of priority. A few nomenclature examples are provided for each of the functional groups. Examples of the various functional groups are presented on pages 115-135 in the textbook. Two overview pages are on pages 136-137. Some functional groups have a suffix name when they are the highest priority functional group and a prefix name when they are not the highest priority group, and these are added to the skeletal names with identifying numbers and stereochemistry terms (E and Z for alkenes, R and S for chiral centers and cis and trans for rings). Several low priority functional groups only have a prefix name. A few additional special patterns are shown on pages 98-102. The only way to learn this topic is practice (over and over). The best practice approach is to actually write out the names (on an extra piece of paper or on a white board, and then do it again). The same functional groups are used throughout the entire course. -

Poly(Arylene Ether)S with Truly Pendant Benzene Sulfonic Acid Groups
Wright State University CORE Scholar Browse all Theses and Dissertations Theses and Dissertations 2008 Poly(arylene ether)s with Truly Pendant Benzene Sulfonic Acid Groups Mohamed Moustafa Abdellatif Wright State University Follow this and additional works at: https://corescholar.libraries.wright.edu/etd_all Part of the Chemistry Commons Repository Citation Abdellatif, Mohamed Moustafa, "Poly(arylene ether)s with Truly Pendant Benzene Sulfonic Acid Groups" (2008). Browse all Theses and Dissertations. 877. https://corescholar.libraries.wright.edu/etd_all/877 This Thesis is brought to you for free and open access by the Theses and Dissertations at CORE Scholar. It has been accepted for inclusion in Browse all Theses and Dissertations by an authorized administrator of CORE Scholar. For more information, please contact [email protected]. POLY(ARYLENE ETHER)S WITH TRULY PENDANT BENZENE SULFONIC ACID GROUPS A thesis submitted in partial fulfillment Of the requirements for the degree of Master of Science By MOHAMED ABDELLATIF B.S. Wright State University, 2006 2008 Wright State University WRIGHT STATE UNIVERSITY SCHOOL OF GRADUATE STUDIES September 4, 2008 I HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPERVISION BY Mohamed Abdellatif ENTITLED Poly(arlyene ether)s with Truly Pendant Benzene Sulfonic Acid Groups BE ACCEPTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Master of Science . ______________________________ Eric Fossum, Ph.D. Thesis Director ______________________________ Kenneth Turnbull, Ph.D. Department Chair Committee on Final Examination _________________________________ Eric Fossum, Ph.D. _________________________________ Kenneth Turnbull, Ph.D. _________________________________ Daniel M. Ketcha, Ph.D. _________________________________ Joseph F. Thomas, Jr., Ph.D. Dean, School of Graduate Studies ABSTRACT Abdellatif, M. -

Chapter 19 the Chemistry of Aldehydes and Ketones. Addition Reactions
Instructor Supplemental Solutions to Problems © 2010 Roberts and Company Publishers Chapter 19 The Chemistry of Aldehydes and Ketones. Addition Reactions Solutions to In-Text Problems 19.1 (b) (d) (e) (g) 19.2 (a) 2-Propanone (d) (E)-3-Ethoxy-2-propenal (f) 4,4-Dimethyl-2,5-cyclohexadienone 19.3 (b) 2-Cyclohexenone has a lower carbonyl stretching frequency because its two double bonds are conjugated. 19.4 (b) The compound is 2-butanone: (c) The high frequency of the carbonyl absorption suggests a strained ring. (See Eq. 19.4, text p. 897.) In fact, cyclobutanone matches the IR stretching frequency perfectly and the NMR fits as well: 19.6 The structure and CMR assignments of 2-ethylbutanal are shown below. The two methyl groups are chemically equivalent, and the two methylene groups are chemically equivalent; all carbons with different CMR chemical shifts are chemically nonequivalent. INSTRUCTOR SUPPLEMENTAL SOLUTIONS TO PROBLEMS • CHAPTER 19 2 19.7 (a) The double bonds in 2-cyclohexenone are conjugated, but the double bonds in 3-cyclohexenone are not. Consequently, 2-cyclohexenone has the UV spectrum with the greater lmax. 19.9 Compound A, vanillin, should have a p T p* absorption at a greater lmax when dissolved in NaOH solution because the resulting phenolate can delocalize into the carboxaldehyde group; the resulting phenolate from compound B, isovanillin, on the other hand, can only delocalize in the aromatic ring. 19.11 The mass spectrum of 2-heptanone should have major peaks at m/z = 43 (from a-cleavage), 71 (from inductive cleavage), and 58 (from McLafferty rearrangement). -

Hot Topics in Neural Membrane Lipidology
A.A. Farooqui Hot Topics in Neural Membrane Lipidology ▶ Focuses exclusively on lipid mediators and neurological disorders Not only do glycerophospholipid, sphingolipid, and cholesterol-derived lipid mediators facilitate the transfer of messages from one cell to another, they also facilitate communication among subcellular organelles. Hot Topics in Neural Membrane Lipidology provides readers with a cutting-edge, comprehensive review of these lipid mediators, their roles and association with neurological disorders, and the future direction of research on the topic. This monograph provides readers with critical data and is particularly accessible to neuroscience graduate students, teachers, and researchers. It can be used as a supplemental text for a range of neuroscience courses. Clinicians and pharmacologists will find this book useful for understanding molecular aspects of lipid mediators in acute neural trauma like stroke, spinal cord trauma, head injury, and neurodegenerative diseases such as Alzheimer disease, Parkinson disease, and Huntington disease. 2009, XXIV, 408 p. 62 illus. About the Author: Printed book Akhlaq A. Farooqui is a leader in the field of brain phospholipases A2, bioactive ether lipid metabolism, and glutamate-mediated neurotoxicity. He has discovered the stimulation of Hardcover plasmalogen-selective phospholipase A2 activity in brains from patients with Alzheimer ▶ 199,99 € | £179.99 | $249.99 disease, and published cutting edge research on the generation and identification of ▶ *213,99 € (D) | 219,99 € (A) | CHF 236.00 glycerophospholipid, sphingolipid, and cholesterol-derived lipid mediators in kainic acid neurotoxicity. Dr. Farooqui has authored three monographs: Glycerophospholipids eBook in Brain: Phospholipase A2 in Neurological Disorders (2007); Neurochemical Aspects of Excitotoxicity (2008); and Metabolism and Functions of Bioactive Ether Lipids in Brain Available from your bookstore or (2008). -

The Ether Lipid-Deficient Mouse: Tracking Down Plasmalogen Functions ⁎ Karin Gorgas A, Andre Teigler B, Dorde Komljenovic A, Wilhelm W
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Biochimica et Biophysica Acta 1763 (2006) 1511–1526 www.elsevier.com/locate/bbamcr Review The ether lipid-deficient mouse: Tracking down plasmalogen functions ⁎ Karin Gorgas a, Andre Teigler b, Dorde Komljenovic a, Wilhelm W. Just b, a Institut für Anatomie und Zellbiologie, Abteilung Medizinische Zellbiologie, Im Neuenheimer Feld 307, D-69120 Heidelberg, Germany b Biochemie-Zentrum der Universität Heidelberg (BZH), Im Neuenheimer Feld 328, D-69120 Heidelberg, Germany Received 29 June 2006; received in revised form 15 August 2006; accepted 23 August 2006 Available online 30 August 2006 Abstract Chemical and physico-chemical properties as well as physiological functions of major mammalian ether-linked glycerolipids, including plasmalogens were reviewed. Their chemical structures were described and their effect on membrane fluidity and membrane fusion discussed. The recent generation of mouse models with ether lipid deficiency offered the possibility to study ether lipid and particularly plasmalogen functions in vivo. Ether lipid-deficient mice revealed severe phenotypic alterations, including arrest of spermatogenesis, development of cataract and defects in central nervous system myelination. In several cell culture systems lack of plasmalogens impaired intracellular cholesterol distribution affecting plasma membrane functions and structural changes of ER and Golgi cisternae. Based on these phenotypic anomalies that were accurately described conclusions were drawn on putative functions of plasmalogens. These functions were related to cell–cell or cell–extracellular matrix interactions, formation of lipid raft microdomains and intracellular cholesterol homeostasis. There are several human disorders, such as Zellweger syndrome, rhizomelic chondrodysplasia punctata, Alzheimer’s disease, Down syndrome, and Niemann–Pick type C disease that are distinguished by altered tissue plasmalogen concentrations.