Educational Research Applications Abebe M, Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Structures of Monosaccharides Hemiacetals
Structures of Monosaccharides Hemiacetals • Although, the open chain structures of monosaccharides are consistent with the chemistry of carbohydrates, in reality they are oversimplifications of the true structure of carbohydrates. • It is common knowledge that aldehydes react with alcohols to form hemiacetals. In cases where a molecule is a hydroxyaldehyde such as 4- hydroxybutanal or 5-hydroxypentanal, cyclic hemiacetals result. 9:47 AM 1 Structures of Monosaccharides Hemiacetals • Aldoses often contain an aldehyde group and several hydroxyl groups as part of the same molecule; they have a greater tendency of forming cyclic hemiacetals. In fact, in aqueous solution carbohydrates exist almost exclusively in the ring-closed form At equilibrium, the linear aldehyde or ketone structure represents less than 1% of the sugar present. • Five and six-membered rings are thermodynamically more stable than their corresponding four and seven membered rings, since they are less strained. • Five- (furanoses) and six-membered cyclic hemiacetals (pyranoses) are often more stable than their open-chain forms. In particular the six-membered rings which can adopt a chair conformation are 9:47 AM 2 essentially free from all types of strains. Structures of Monosaccharides Evidence for Existence of Monosacharides as Hemiacetals What physical, chemical and spectroscopic evidence support the existence of monosaccharide sugars as cyclic hemi-acetals. (a) Two anomers of glucose capable of existing independently with different physical (melting points and specific optical rotation) and chemical properties can be obtained by recrystallization. (b) the 1H-NMR and IR-spectra of solutions of pure sugars show the presence of mixtures (anomeric hemiacetals) and absence of an aldehydic peak is a sufficient indicator that the sugars exist in some other form other than the open-chain form. -

Aldehydes Can React with Alcohols to Form Hemiacetals
340 14 . Nucleophilic substitution at C=O with loss of carbonyl oxygen You have, in fact, already met some reactions in which the carbonyl oxygen atom can be lost, but you probably didn’t notice at the time. The equilibrium between an aldehyde or ketone and its hydrate (p. 000) is one such reaction. O HO OH H2O + R1 R2 R1 R2 When the hydrate reverts to starting materials, either of its two oxygen atoms must leave: one OPh came from the water and one from the carbonyl group, so 50% of the time the oxygen atom that belonged to the carbonyl group will be lost. Usually, this is of no consequence, but it can be useful. O For example, in 1968 some chemists studying the reactions that take place inside mass spectrometers needed to label the carbonyl oxygen atom of this ketone with the isotope 18 O. 16 18 By stirring the ‘normal’ O compound with a large excess of isotopically labelled water, H 2 O, for a few hours in the presence of a drop of acid they were able to make the required labelled com- í In Chapter 13 we saw this way of pound. Without the acid catalyst, the exchange is very slow. Acid catalysis speeds the reaction up by making a reaction go faster by raising making the carbonyl group more electrophilic so that equilibrium is reached more quickly. The the energy of the starting material. We 18 also saw that the position of an equilibrium is controlled by mass action— O is in large excess. -

In This Handout, All of Our Functional Groups Are Presented As Condensed Line Formulas, 2D and 3D Formulas and with Nomenclature Prefixes and Suffixes (If Present)
In this handout, all of our functional groups are presented as condensed line formulas, 2D and 3D formulas and with nomenclature prefixes and suffixes (if present). Organic names are built on a foundation of alkanes, alkenes and alkynes. Those examples are presented first and you need to know those rules. The strategies can be found in Chapter 4 of our textbook (alkanes: pages 93-98, cycloalkanes 102-104, alkenes: pages 104-110, alkynes: pages 112-113 and combinations of all of them 113-115). After introducing examples of alkanes, alkenes, alkynes and combinations of them, the functional groups are presented in order of priority. A few nomenclature examples are provided for each of the functional groups. Examples of the various functional groups are presented on pages 115-135 in the textbook. Two overview pages are on pages 136-137. Some functional groups have a suffix name when they are the highest priority functional group and a prefix name when they are not the highest priority group, and these are added to the skeletal names with identifying numbers and stereochemistry terms (E and Z for alkenes, R and S for chiral centers and cis and trans for rings). Several low priority functional groups only have a prefix name. A few additional special patterns are shown on pages 98-102. The only way to learn this topic is practice (over and over). The best practice approach is to actually write out the names (on an extra piece of paper or on a white board, and then do it again). The same functional groups are used throughout the entire course. -

Structures of Monosaccharides Hemiacetals
Disaccharides 10:51 AM 1 Disaccharides Definition • Disaccharides are carbohydrates consisting of two monosaccharide units linked via a glycosidic bond. Non-reducing disaccharide (1,1'-Glycosidic linkage) OH HO OH O HO O OH O OH OH HO OH HO O O HO OH + HO OH Glycosidic bond OH OH HO OH HO OH 6' 6 O O Reducing end 5' 1' 4 5 HO 4' O OH 3' 2' 3 2 1 HO OH HO OH Glycone Aglycone Reducing disaccharide (1,4'-Glycosidic linkage) • These disaccharides may be reducing or non-reducing sugars depending on the regiochemistry of the glycosidic 10:51 AM linkage between the two monosaccharides. 2 Nomenclature of Disaccharides • Since disaccharides are glycosides with two monosaccharide units linked through a glycosidic bond, their nomenclature requires the formulation of priority rules to identify which of the two monosaccharides of a disaccharide provides the parent name of the disaccharide and which one will be considered the substituent. • The nomenclature of disaccharides is based on the following considerations: i. Disaccharides with a free hemiacetal group (Reducing disaccharide) ii. Disaccharides without a free hemiacetal group (Non- Reducing Disaccharide) 10:51 AM 3 Nomenclature of Reducing Disaccharides • A disaccharide in which one glycosyl unit appears to have replaced the hydrogen atom of a hydroxyl group of the other is named as a glycosylglycose. The locants of the glycosidic linkage and the anomeric descriptor(s) must be given in the full name. • The parent sugar residue in such a reducing disaccharide is chosen on the basis of the following criteria: • The parent sugar residue is the one that includes the functional group most preferred by general principles of organic nomenclature. -

Chapter 16 ! Bonds with Hidden Leaving Groups (Sections 16.1-16.6 Excluding 16.3.1 and 16.5.3 ) O Formation and Reactivity of Acetals
O Chemistry 2600 Chapter 16 ! Bonds With Hidden Leaving Groups (sections 16.1-16.6 excluding 16.3.1 and 16.5.3 ) O Formation and Reactivity of Acetals • In Chapters 7 and 15 we saw how carbonyl compounds undergo addition reactions with nucleophiles. • We also saw how some of these reactions are reversible. • For example, recall the formation of a hydrate when an aldehyde or ketone reacts with water under acidic condition: 2 O Formation and Reactivity of Acetals • This reaction is reversible and after the loss of a leaving group, the carbonyl group is reformed: • Note that the oxygen that is lost could come from the initial carbonyl oxygen atom. • We say these molecules have a ‘hidden leaving group’. • This chapter explores the chemistry of the removal or replacement of these hidden leaving groups. 3 O Formation and Reactivity of Acetals • When an aldehyde/ketone reacts with an alcohol in the presence of acid, a hemiacetal is formed. • The only difference between hemiacetal formation and hydrate formation is the nucleophile (water vs alcohol). • Hemiacetals have a hidden leaving group and in the presence of alcohol and acid, react quickly to form an acetal. • Hemiacetal (tetrahedral carbon attached to –OH and –OR) • Acetal (tetrahedral carbon attached to two –OR groups) 4 O Formation and Reactivity of Acetals • Acetal formation: 5 O Formation and Reactivity of Acetals • Acetal formation is an equilibrium process, all the steps in the sequence can run in both directions. • When an acetal is converted to a carbonyl compound, the reaction is called hydrolysis because water is being used to break (lyse) the acetal. -

Chapter 16: Carboxylic Acids, Esters, and Other Acid Derivatives
Chapter 16: Carboxylic Acids, Esters, and Other Acid Derivatives In Chapter 15, we discussed the carbonyl group and two families of compounds -aldehydes and ketones—that contain C=O group. In this chapter, we discuss four more families of compounds in which the carbonyl group is present: a) carboxylic acid, b) esters, c) amides, d) acid chlorides, and e) acid anhydrides and f) carboxylic acid salts. 16.1 Structure of Carboxylic Acids and Their Derivatives A carboxylic acid is an organic compound whose functional group is the carboxyl group. What is a carboxyl group? A carboxyl group is a carbonyl group (C=O) with a hydroxyl group (—OH) bonded to the carboxyl carbon atom. A general structural representation fit a carboxyl group is Abbreviated linear designations for the carboxyl group are Although we see within a carboxyl group both a carbonyl group (C=O) and hydroxyl group (—OH). 16.2 IUPAC Nomenclature for Carboxylic Acids The naming of carboxylic acids is fairly simple. You simply find the longest carbon chain which includes the carboxylic group. Use that as the stem for the name, cross off the -e on the ending of the alkane name and replace it with -oic acid. I think you can see how that works, if you look at this example (which is also shown in Example 1-a in your workbook). It gives you, in this case propanoic acid (with a three-carbon-atom chain), the (from propan + oic acid) name propanoic acid. As with aldehydes, it is not necessary to indicate where the acid functional group is because it has to be at the end of the molecule, on the #1 carbon. -
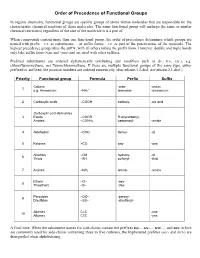
Priority of Functional Groups
Order of Precedence of Functional Groups In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a part of. When compounds contain more than one functional group, the order of precedence determines which groups are named with prefix – i.e. as substituents –, or suffix forms – i.e. as part of the parent name of the molecule. The highest precedence group takes the suffix, with all others taking the prefix form. However, double and triple bonds only take suffix form (-ene and -yne) and are used with other suffixes. Prefixed substituents are ordered alphabetically (excluding any modifiers such as di-, tri-, etc.), e.g. chlorofluoromethane, not fluorochloromethane. If there are multiple functional groups of the same type, either prefixed or suffixed, the position numbers are ordered numerically (thus ethane-1,2-diol, not ethane-2,1-diol.) Priority Functional group Formula Prefix Suffix Cations -onio- -onium 1 + e.g. Ammonium –NH4 ammonio- -ammonium 2 Carboxylic acids –COOH carboxy- -oic acid Carboxylic acid derivatives 3 Esters –COOR R-oxycarbonyl- Amides –CONH2 carbamoyl- -amide 4 Aldehydes –CHO formyl- -al 5 Ketones >CO oxo- -one Alcohols –OH hydroxy- -ol 6 Thiols –SH sulfanyl- -thiol 7 Amines –NH2 amino- -amine Ethers –O– -oxy- 8 Thioethers –S– -thio- Peroxides –OO– -peroxy- 9 Disulfides –SS– -disulfanyl- Alkenes C=C -ene 10 Alkynes C≡C -yne A final note: When the substituent names for side-chains contain the prefixes iso..., sec-..., tert-..., and neo (which are commonly used for side-chains containing three to five carbons), the hyphenated prefixes (sec- and tert-) are disregarded when alphabetizing. -

1 Chapter 3: Organic Compounds: Alkanes and Cycloalkanes
Chapter 3: Organic Compounds: Alkanes and Cycloalkanes >11 million organic compounds which are classified into families according to structure and reactivity Functional Group (FG): group of atoms which are part of a large molecule that have characteristic chemical behavior. FG’s behave similarly in every molecule they are part of. The chemistry of the organic molecule is defined by the function groups it contains 1 C C Alkanes Carbon - Carbon Multiple Bonds Carbon-heteroatom single bonds basic C N C C C X X= F, Cl, Br, I amines Alkenes Alkyl Halide H C C C O C C O Alkynes alcohols ethers acidic H H H C S C C C C S C C H sulfides C C thiols (disulfides) H H Arenes Carbonyl-oxygen double bonds (carbonyls) Carbon-nitrogen multiple bonds acidic basic O O O N H C H C O C Cl imine (Schiff base) aldehyde carboxylic acid acid chloride O O O O C C N C C C C O O C C nitrile (cyano group) ketones ester anhydrides O C N amide opsin Lys-NH2 + Lys- opsin H O H N rhodopsin H 2 Alkanes and Alkane Isomers Alkanes: organic compounds with only C-C and C-H single (s) bonds. general formula for alkanes: CnH(2n+2) Saturated hydrocarbons Hydrocarbons: contains only carbon and hydrogen Saturated" contains only single bonds Isomers: compounds with the same chemical formula, but different arrangement of atoms Constitutional isomer: have different connectivities (not limited to alkanes) C H O C4H10 C5H12 2 6 O OH butanol diethyl ether straight-chain or normal hydrocarbons branched hydrocarbons n-butane n-pentane Systematic Nomenclature (IUPAC System) Prefix-Parent-Suffix -

20H-Carbohydrates.Pdf
Carbohydrates Carbohydrates are compounds that have the general formula CnH2nOn Because CnH2nOn can also be written Cn(H2O)n, they appear to be “hydrates of carbon” Carbohydrates are also called “sugars” or “saccharides” Carbohydrates can be either aldoses (ald is for aldehyde and ose means a carbohydrate) or ketoses (ket is for ketone) OH OH O OH CH2OH CH2OH OHC HOH2C OH OH OH OH An Aldose A Ketose (D-Glucose) (D-Fructose) Carbohydrates Due to the multiple chiral centers along a linear carbon chain for carbohydrates, Emil Fischer developed the “Fischer Projection” in order to represent these compounds Remember how to draw a Fischer projection: 1) View the linear carbon chain along the vertical axis (always place the more oxidized carbon [aldehyde in an aldose] towards the top) 2) The horizontal lines are coming out of the page toward the viewer 3) Will need to change the viewpoint for each carbon so the horizontal substituents are always pointing towards the viewer CHO OH OH H OH HO H CH2OH = OHC H OH OH OH H OH CH2OH Emil Fischer (1852-1919) Carbohydrates The aldoses are thus all related by having an aldehyde group at one end, a primary alcohol group at the other end, and the two ends connected by a series of H-C-OH groups CHO CHO CHO CHO CHO H OH H OH H OH H OH HO H CH2OH H OH H OH H OH HO H CH2OH H OH H OH HO H CH2OH H OH HO H CH2OH CH2OH Aldotriose Aldotetrose Aldopentose Aldohexose Aldohexose D-glyceraldehyde D-erythose D-ribose D-allose L-allose The D-aldoses are named according to glyceraldehyde, the D refers to the configurational -

Ch.04Carbon and the Molecular Diversity of Life
EXPERIMENT “Atmosphere” CH4 Water vapor Electrode Molecular Structural Ball-and-Stick Space-Filling Name Formula Formula Model Model NH 3 H 2 (a) Methane Condenser Cooled water (b) Ethane containing Cold organic water molecules (c) Ethene (ethylene) H2O “sea” Sample for chemical analysis 1 2 Hydrogen Oxygen Nitrogen Carbon (valence = 1) (valence = 2) (valence = 3) (valence = 4) H O N C Urea 3 4 Fat droplets (stained red) Ethane Propane 1-Butene 2-Butene (a) Length (c) Double bonds Butane 2-Methylpropane (commonly called isobutane) Cyclohexane Benzene (b) Branching (d) Rings 100 µm (a) Mammalian adipose cells (b) A fat molecule 5 6 Effective Ineffective Drug Condition Pentane 2-methyl butane Enantiomer Enantiomer (a) Structural isomers Ibuprofen Pain; inflammation cis isomer: The two Xs are trans isomer: The two Xs are S-Ibuprofen R-Ibuprofen on the same side. on opposite sides. (b) Geometric isomers Albuterol Asthma R-Albuterol S-Albuterol L isomer D isomer (c) Enantiomers 7 8 Estradiol Testosterone L-dopa D-dopa 9 10 CHEMICAL Hydroxyl Carbonyl Carboxyl CHEMICAL Amino Sulfhydryl Phosphate Methyl GROUP GROUP (may be STRUCTURE STRUCTURE written HS—) (may be written HO—) The amino group The sulfhydryl group In a phosphate group, a A methyl group consists of a In a hydroxyl group (—OH), a The carbonyl group ( CO) When an oxygen atom is (—NH2) consists of a consists of a sulfur atom phosphorus atom is bonded to carbon bonded to three hydrogen atom is bonded to an consists of a carbon atom double-bonded to a carbon nitrogen atom bonded bonded to an atom of four oxygen atoms; one oxygen hydrogen atoms. -

Chapter 3 Alcohols, Phenols, and Ethers
Chapter 3 Alcohols, Phenols, and Ethers Chapter 3 Alcohols, Phenols, and Ethers Chapter Objectives: • Learn to recognize the alcohol, phenol, and ether functional groups. • Learn the IUPAC system for naming alcohols, phenols, and ethers. • Learn the important physical properties of the alcohols, phenols, and ethers. • Learn the major chemical reaction of alcohols, and learn how to predict the products of dehydration and oxidation reactions. • Learn to recognize the thiol functional group. Mr. Kevin A. Boudreaux Angelo State University CHEM 2353 Fundamentals of Organic Chemistry Organic and Biochemistry for Today (Seager & Slabaugh) www.angelo.edu/faculty/kboudrea Introduction • In this chapter, we will start looking at organic molecules that incorporate C—O bonds. • Oxygen is in Group 6A of the periodic table, and in most of its compounds, contains two single bonds and two lone pairs (or one double bond and two lone pairs), and is sp3-hybridized with a bent molecular shape: O O •The alcohol, phenol, and ether functional groups are found in a number of important naturally occurring molecules: CH3CH2OH Ethanol OH CH3CH2OCH2CH3 Diethyl ether HO Menthol Cholesterol 2 1 Chapter 3 Alcohols, Phenols, and Ethers Alcohols 3 The Hydroxy (—OH) Functional Group •The hydroxyl group (—OH) is found in the alcohol and phenol functional groups. (Note: that’s not the same as hydroxide, OH-, which is ionic.) –in alcohols, a hydroxyl group is connected to a carbon atom. –in phenols, —OH is connected to a benzene ring. (The “parent” molecule of this class is also named phenol: PhOH or C6H5OH.) • When two carbon groups are connected by single bonds to an oxygen, this is classified as the ether functional group. -

1 General Aspects of the Glycosidic Bond Formation Alexei V
j1 1 General Aspects of the Glycosidic Bond Formation Alexei V. Demchenko 1.1 Introduction Since the first attempts at the turn of the twentieth century, enormous progress has been made in the area of the chemical synthesis of O-glycosides. However, it was only in the past two decades that the scientificworldhadwitnessedadramatic improvement the methods used for chemical glycosylation. The development of new classes of glycosyl donors has not only allowed accessing novel types of glycosidic linkages but also led to the discovery of rapid and convergent strategies for expeditious oligosaccharide synthesis. This chapter summarizes major prin- ciples of the glycosidic bond formation and strategies to obtain certain classes of compounds, ranging from glycosides of uncommon sugars to complex oligosac- charide sequences. 1.2 Major Types of O-Glycosidic Linkages There are two major types of O-glycosides, which are, depending on nomen- clature, most commonly defined as a-andb-, or 1,2-cis and 1,2-trans glycosides. The 1,2-cis glycosyl residues, a-glycosides for D-glucose, D-galactose, L-fucose, D-xylose or b-glycosides for D-mannose, L-arabinose, as well as their 1,2-trans counter- parts (b-glycosides for D-glucose, D-galactose, a-glycosides for D-mannose,etc.),are equally important components in a variety of natural compounds. Representative examples of common glycosides are shown in Figure 1.1. Some other types of glycosides, in particular 2-deoxyglycosides and sialosides, can be defined neither as 1,2-cis nor as 1,2-trans derivatives, yet are important targets because of their com- mon occurrence as components of many classes of natural glycostructures.