Oxidation of Alcohols
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Studies Toward Synthesis of Polycyclic Polyprenylated Acylphloroglucinols
University of Kentucky UKnowledge University of Kentucky Doctoral Dissertations Graduate School 2006 STUDIES TOWARD SYNTHESIS OF POLYCYCLIC POLYPRENYLATED ACYLPHLOROGLUCINOLS Roxana Ciochina University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Ciochina, Roxana, "STUDIES TOWARD SYNTHESIS OF POLYCYCLIC POLYPRENYLATED ACYLPHLOROGLUCINOLS" (2006). University of Kentucky Doctoral Dissertations. 291. https://uknowledge.uky.edu/gradschool_diss/291 This Dissertation is brought to you for free and open access by the Graduate School at UKnowledge. It has been accepted for inclusion in University of Kentucky Doctoral Dissertations by an authorized administrator of UKnowledge. For more information, please contact [email protected]. ABSTRACT OF DISSERTATION Roxana Ciochina The Graduate School University of Kentucky 2006 STUDIES TOWARD SYNTHESIS OF POLYCYCLIC POLYPRENYLATED ACYLPHLOROGLUCINOLS ABSTRACT OF DISSERTATION A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the College of Arts and Sciences at the University of Kentucky By Roxana Ciochina Lexington, KY Director: Dr. R. B. Grossman, Professor of Chemistry Lexington, KY 2006 ABSTRACT OF DISSERTATION STUDIES TOWARD SYNTHESIS OF POLYCYCLIC POLYPRENYLATED ACYLPHLOROGLUCINOLS Polycyclic polyprenylated acylphloroglucinols (PPAPs) are a class of compounds that reveal intriguing biological activities and interesting and challenging chemical structures. These products are claimed to possess antioxidant, antiviral, and antimitotic properties. Increasing interest is related to their function in the CNS as modulators of neurotransmitters associated to neuronal damaging and depression. All these features make PPAPs targets for synthesis. We decided to focus our own initial efforts in this area on the type A PPAP, nemorosone because we thought that its fairly simple structure relative to other PPAPs would present fewer hurdles as we developed our methodology. -

Chemistry 301-301A - Hour Examination #3, December 11, 2003
Chemistry 301-301A - Hour Examination #3, December 11, 2003 “.....as we know, there are known unknowns; there are things we know we know. We also know there are known unknowns; that is to say we know there are some things we do not know. But there are also unknown unknowns - the ones we don't know we don't know.” Donald Rumsfeld (winner of a British award given to the worst mangler of the English language in 2003) “I know, a proof is a proof. What kind of a proof is a proof? A proof is a proof and when you have a good proof it's because it's proven." Jean Chrétien (hon. mention for the same award) 1[18 points] (a) Acid-catalyzed addition of water to 3-methyl-1-butene (1) results in formation of large amounts of a rearranged alcohol (2), in addition to the expected alcohol (3). Explain, with excellent arrow formalisms. H2O + H O+ 3 OH OH 1 3 2 (b) On the other hand hydroboration of 1, followed by oxidation, does not lead to any rearranged product. Only alcohol 4 is formed. Explain. Detailed mechanisms are not needed here, but a drawing of the transition state for the hydroboration step is. 1. BH3 OH 2. HOOH/HO – 1 4 (c) But there are some strange things that happen in hydroboration. For example when 2-methyl-2-butene (5) is hydroborated at high temperature, then treated with HOOH/HO–, alcohol 4 is still one of the products. Explain mechanistcally. Hint: at high temperature hydroboration is reversible. -

The Mechanism of the Baeyer–Villiger Rearrangement: Quantum Chemistry and TST Study Supported by Experimental Kinetic Data†
PAPER www.rsc.org/obc | Organic & Biomolecular Chemistry The mechanism of the Baeyer–Villiger rearrangement: quantum chemistry and TST study supported by experimental kinetic data† J. Raul Alvarez-Idaboy,*a Lino Reyesa and Nelaine Mora-Diezb Received 15th August 2007, Accepted 19th September 2007 First published as an Advance Article on the web 2nd October 2007 DOI: 10.1039/b712608e The mechanism of the Baeyer–Villiger rearrangement is modelled for the reaction of propanone with trifluoroperacetic acid, catalyzed by trifluoroacetic acid in dichloromethane, using three DFT methods (B3LYP, BH&HLYP and MPWB1K) and MP2. These results are refined and used to calculate the overall reaction rate coefficient using conventional Transition State Theory. The excellent agreement between the calculated (1.00 × 10−3 L mol−1 s−1) and the experimental (1.8 × 10−3 L mol−1 s−1)rate coefficients at the MPWB1K level strongly supports the mechanism recently proposed by our group. This DFT method is then used to study the mechanism of a larger system: cyclohexanone + trifluoroperacetic acid, for which a very good agreement between the calculated and the experimental rate coefficients is also found (1.37 and 0.32 L mol−1 s−1, respectively). The modelled mechanism is not ionic but neutral, and consists of two concerted steps. The first one is strongly catalyzed while the second one, the migration step, seems not to be catalyzed for the systems under study. The results of this work could be of interest for understanding other reactions in non-polar solvents for which ionic mechanisms have been assumed. -

Some Aspects the Baeyer-Villicer Reaction
SOME ASPECTS THE BAEYER-VILLICER REACTION - by - JOHN EDVARD BOLLIG-ER. B.So, CSyd.) A. Thesis submitted for the degree of MASTER OF SCIENCE - at the - UNIVERSITY OP NEW SOUTH WALES April, 1963- CONTENTS Page No. Summary . .. 1 The Baeyer-Villiger Reaction .. 2 Discussion: .. .. 26 Baeyer-Villiger Oxidation of 2-Bromocholestan-3-one 32 Baeyer-Villiger Oxidation of 2-Bromofriedelin 50 Baeyer-Villiger Oxidation of 2-Chlorocholestan-3-one 53 Baeyer-Villiger Oxidation of 2-Iodocholestan-3-one 54 Baeyer-Villiger Oxidation of Gerin 56 Experimental .. .. 61 Acknowledgments .. .. 87 Bibliography .. .. 88 1 SUMMARY V/ith a view to synthesising suitable inter mediates for intramolecular Darzen's glycidic ester syntheses, certain steroid and triterpenoid a- substituted ketones have been subjected to Baeyer- Yilliger oxidation. In some cases the oxidation yielded unexpected products and possible mechanisms for their formation are discussed. In other cases the expected products were obtained but readily under went an unusual rearrangement. The structures of the rearranged products have been chemically elucidated and the mechanism of the rearrangement is discussed. This thesis is prefaced by a discussion of the Baeyer-Villiger reaction. THE BAEYER-VTLLIGER REACTION The reaction of ketones with peracids to give esters was first observed by Baeyer and Villiger in 1899 . The scope of this reaction, now known as the Baeyer-Yilliger reaction, has since been widely extended and it has found many useful applications in organic chemistry. Among its representative uses, illustrated by many examples in p the literature , are the formation of esters from simple aliphatic or aromatic ketones, formate esters from aldehydes, anhydrides from o—diketones, lactones from alicyclic ketones and enol lactones from a,0-unsaturated alicyclic ketones. -

Synthesis of Alkynyl Ribofuranosides
City University of New York (CUNY) CUNY Academic Works Dissertations and Theses City College of New York 2011 Synthesis of Alkynyl Ribofuranosides Christian Rodriguez CUNY City College How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/cc_etds_theses/25 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] SYNTHESIS OF ALKYNYL RIBOFURANOSIDES A Thesis Presented to The Faculty of the Chemistry Program The City College of New York In (Partial) Fulfillment of the Requirements for the Degree Master of Arts by Christian Rodriguez December, 2010 - 1 - Synthesis of Alkynyl Ribofuranosides By Christian Rodriguez Mentor: P. Meleties Table of Contents Chapter 1 1.1 Introduction 6 1.2 Preparation of ribonolactone template 8 1.3 Synthesis of protected ribonolactone 9 Chapter 2 2.1 Preparation of ethynyl ribofuranosides 10 2.2 Reaction with ethynylmagnesium bromide 12 2.3 Intramolecular cyclization of diyne diol 14 2.4 Reaction of ribonolactone with lithium acetylide 15 2.5 Boron trifluoride hemiacetal deoxygenation 15 2.6 Alternative deacetylation 16 Chapter 3 3.1 Selecting appropriate protecting group 19 3.2 Preparation of trimethylsilyl alkynyl 5-O-benzyl-2,3-O isopropylidene 19 ribofuranoside 3.3 Lewis acid promoted triethylsilane dehydroxylation mechanism 20 Chapter 4 4.1 Hemiacetal alkynylation 25 4.2 Hemiacetal nucleophilic addition mechanism 26 4.3 Intramolecular -
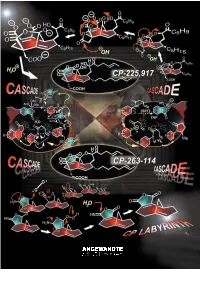
A Paradigm of How Endeavors in Total Synthesis Lead to Discoveries and Inventions in Organic Synthesis
REVIEWS The CP Molecule Labyrinth: A Paradigm of How Endeavors in Total Synthesis Lead to Discoveries and Inventions in Organic Synthesis K. C. Nicolaou* and Phil S. Baran Dedicated to Mrs. Niki Goulandris for her outstanding contributions to humanity and Planet Earth on the occasion of the opening of the GAIA Center for Environmental Research and Education at the Goulandris Natural History Museum in Athens, Greece. Imagine an artist carving a sculpture Herculean nature of the task and the ed Minotaur, which he accomplished from a marble slab and finding gold rewards that accompany it, one must through brilliance, skill, and bravery nuggets in the process. This thought is sense the details of the enterprise having traversed the famous labyrinth not a far-fetched description of the behind the scenes. A more vivid de- with the help of Ariadne. This story work of a synthetic chemist pursuing scription of total synthesis as a struggle from Greek mythology comes alive in the total synthesis of a natural product. against a tough opponent is perhaps modern synthetic expeditions toward At the end of the day, he or she will be appropriate to dramatize these ele- natural products as exemplified by the judged by the artistry of the final work ments of the experience. In this article total synthesis of the CP molecules and the weight of the gold discovered we describe one such endeavor of total which serve as a paradigm for modern in the process. However, as colorful as synthesis which, in addition to reaching total synthesis endeavors, where the this description of total synthesis may the target molecule, resulted in a objectives are discovery and invention be, it does not entirely capture the wealth of new synthetic strategies and in the broader sense of organic syn- essence of the endeavor, for there is technologies for chemical synthesis. -

Radical Hydroacylation of C-C and N-N Double Bonds in Air
University College London Radical Hydroacylation of C-C and N-N Double Bonds in Air by Jenna Marie Ahern Submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy Declaration I, Jenna Marie Ahern, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. Jenna Marie Ahern October 2010 Radical Hydroacylation of C-C and N-N Double Bonds in Air Jenna Marie Ahern Abstract The formation of C-C and C-N bonds in modern organic synthesis is a key target for methodological advancement. Current methods of C-C and C-N bond formation often involve the use of expensive catalysts, or sub-stoichiometric reagents, which can lead to the generation of undesirable waste products. This thesis describes a novel and environmentally benign set of reaction conditions for the formation of C-C and C-N bonds by hydroacylation and this is promoted by mixing two reagents, an aldehyde and an electron-deficient double bond, under freely available atmospheric oxygen at room temperature Chapter 1 will provide an introduction to the thesis and mainly discusses methods for C-C bond formation, in particular, radical chemistry and hydroacylation. Chapter 2 describes the hydroacylation of vinyl sulfonates and vinyl sulfones (C-C double bonds) with aliphatic and aromatic aldehydes with a discussion and evidence for the mechanism of the transformation. Chapter 3 details the synthesis of precursors for intramolecular cyclisations and studies into aerobic intramolecular cyclisations. Chapter 4 describes the hydroacylation of vinyl phosphonates (C-C double bonds) and diazocarboxylates (N-N double bonds) with aliphatic and aromatic aldehydes bearing functional groups. -

Synthesis of an Unsymmetrically Pentafunctionalized Corannulene Derivative (Part I) Synthesis of Platinum and Ethynyl-Platinum Corannulenes (Part II)
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2012 Synthesis of an Unsymmetrically Pentafunctionalized Corannulene Derivative (Part I) Synthesis of Platinum and Ethynyl-Platinum Corannulenes (Part II) Maag, Roman M Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-164179 Dissertation Published Version Originally published at: Maag, Roman M. Synthesis of an Unsymmetrically Pentafunctionalized Corannulene Derivative (Part I) Synthesis of Platinum and Ethynyl-Platinum Corannulenes (Part II). 2012, University of Zurich, Faculty of Science. Part I: Synthesis of an Unsymmetrically Pentafunctionalized Corannulene Derivative and Part II: Synthesis of Platinum and Ethynyl-Platinum Corannulenes Dissertation zur Erlangung der naturwissenschaftlichen Doktorwurde¨ Dr. sc. nat. vorgelegt der Mathematisch-naturwissenschaftlichen Fakult¨at der Universit¨at Zurich¨ von Roman M. Maag von Winkel ZH Promotionskommitee: Prof. Dr. Jay S. Siegel (Vorsitz) Prof. Dr. Kim K. Baldridge Prof. Dr. Cristina Nevado Prof. Dr. Roger Alberto Zurich,¨ 2012 Abstract of the Dissertation Part I: Synthesis of an Unsymmetrically Pentafunctionalized Corannulene Derivative and Part II: Synthesis of Platinum and Ethynyl-Platinum Corannulenes by Roman M. Maag University of Zurich, 2012 Prof. Dr. Jay S. Siegel, Chair Corannulene (C20H10) is a polyaromatic hydrocarbon that can be considered as the smallest fragment of Buckminsterfullerene exhibiting a curved surface. Among the in- teresting properties of corannulene are rapid bowl inversion and esthetically appealing fivefold symmetry (C5v), which is rare in chemistry. Whereas the first synthesis in 1968 only afforded milligram quantities, several improvements in the synthetic strategy finally culminated in the development of an efficient process which today furnishes corannulene in kilogram quantities. -

Changxia Yuan Baran Group Meeting 4/5/2014 Commercial Available
Baran Group Meeting Changxia Yuan 4/5/2014 Commercial available peroxides* Inorganic peroxides Na2O2 CaO2 Li2O2 BaO2 Ni2O3 NiO2 xH2O H2O2(30%) ZnO2 NaBO3 4H2O MgO2 TbO2 SrO2 Na2CO3 1.5H2O sodium calcium lithium barium nickel nickel(II) hydrogen zinc sodium magnesium terbium Stronium sodium peroxide peroxide peroxide peroxide peroxide peroxide peroxide peroxide perborate peroxide peroxide peroxide percarbonate $ 109/100g $ 27/100g $ 32 /50g $ 146/500g $ 106/5g hydrate $ 350/4L $ 75/1kg tetrahydrate complex $ 30/1g $ 38/100g $ 91/ 2.5kg $ 40/ 1g $94/1kg $ 40/250g + (NH4)2S2O8 Na2S2O8 K2S2O8 2K2SO5 KHSO4 K2SO4 5[Bu4N ] SO5] HSO4] SO4] ammonium sodium potassium Oxone® OXONE® persulfate persulfate persulfate monopersulfate tetrabutylammonium salt $ 39/ 100g $ 87/ 2.5kg $ 70/ 500g compound $ 156/ 25g $ 60/ 1kg Organic peroxides-1 O CO3H O HOO tBu O O OO O CH2(CH2)9CH3 O H3C(H2C)9H2C O O tBu tBuOOH urea H2O2 BzOOBz OOH O Cl O tert-Butyl Urea Benzoyl mCPBA Cyclobutane 2-Butanone tert-Butyl Lauoyl hydroperoxide hydrogen peroxide $ 81/100g maloyl peroxide peroxide peroxide solution (5-6 M) peroxide $ 92/500g peroxide $ 129/500mL $ 134/1L $ 81/100g $ 47/25mL $ 88/ 250g $ 100/1g Cl O O Cl O Me Me O Me Me Me Me O O O O Ph O O O O OO O Me O Me O Ph O O tBu Cl Ph Me Me Me O Me 2,4-Dichlorobenzoyl Cl tert-Butyl tert-Butyl peracetate solution, Dicumyl tert-Butylperoxy 1,1-Bis(tert-amylperoxy)cyclohexane peroxide, 50% in DBP peroxybenzoate 50% in mineral spirits peroxide 2-ethylhexyl solution, 80% in mineral spirits $ 59/100g $ 86/500mL $ 77/500mL $ 123/500g -

Development of Copper-Catalyzed Chemoselective Reactions
Vol. 68, No. 5 Chem. Pharm. Bull. 68, 405–420 (2020) 405 Review Development of Copper-Catalyzed Chemoselective Reactions Yohei Shimiz u a,b a Department of Chemistry, Faculty of Science, Hokkaido University; Kita 10 Nishi 8, Kita-ku, Sapporo 060–0810, Japan: and b Institute for Chemical Reaction Design and Discovery (WPI- ICReDD), Hokkaido University; Kita 21 Nishi 10, Kita-ku, Sapporo 001–0021, Japan. Received August 30, 2019 Chemoselective reactions can contribute to streamlining synthesis of pharmaceuticals, agrochemicals, and other functional molecules by avoiding use of protecting groups. In this review, copper catalysts were demonstrated useful for developing two types of chemoselective reactions: C–C bond-forming reactions at an anomeric carbon of unprotected aldoses and difunctionalization reaction of C–C multiple bonds. The “soft” nucleophilic copper species exhibit orthogonal reactivity toward “hard” polar functional groups and prefer- entially react with “soft” functional groups. The catalysis also controls stereoselectivity and/or regioselectiv- ity to provide value-added products from readily available feedstock compounds. Key words chemoselective reaction; catalyst; copper; aldose; multiple bond; difunctionalization 1. Introduction carbon nucleophiles to develop chemoselective C–C bond- Biologically active molecules often possess multiple func- forming reactions. The soft organocopper species chemoselec- tional groups. Synthesis of these compounds requires protect- tively reacts with soft carbon electrophiles in the presence of ing groups to secure the promotion of intended transformation polar hard functional groups without protecting these possible at the particular functional group of the starting molecule. reactive sites. In this review we introduce two types of trans- The protecting groups, however, are not included in the final formations: C–C bond-forming reactions at anomeric position products. -

The Role of Acid Catalysis in the Baeyer−Villiger Reaction. a Theoretical Study Robert D
Article pubs.acs.org/joc The Role of Acid Catalysis in the Baeyer−Villiger Reaction. A Theoretical Study Robert D. Bach* Department of Chemistry and Biochemistry, University of Delaware, Newark, Delaware 19716, United States *S Supporting Information ABSTRACT: Quantum mechanical calculations at the B3LYP/6- 311+G(d,p) level have examined the overall mechanism of the Baeyer−Villiger (BV) reaction with peroxyacetic acid. A series of reactions that include both the addition step and the subsequent alkyl group migration step included ketones, acetone, t-butyl methyl ketone, acetophenone, cyclohexyl methyl ketone, and cyclohexyl phenyl ketone. The combined data suggested that the first step for addition of the peroxyacetic acid oxidation catalyst to the ketone carbonyl to produce the Criegee or tetrahedral intermediate is rate- limiting and has activation barriers that range from 38 to 41 kcal/ mol without the aid of a catalyst. The rate of addition is markedly reduced by the catalytic action of a COOH functionality acting as a donor−acceptor group affecting both its proton transfer to the ketone CO oxygen in concert with transfer of the OOH proton to the carboxylic acid carbonyl. The second or alkyl group migration step has a much reduced activation barrier, and its rate is not markedly influenced by acid catalysis. The rate of both steps in the BV reaction is greatly influenced by the catalytic action of very strong acids. ■ INTRODUCTION mechanism for addition to the carbonyl group and how the hemiperacetal hydrogen migrates to the carboxylate to produce The Baeyer−Villiger (BV) reaction remains an important the final products, an ester functionality and a carboxylic acid. -

Application of Complex Aldol Reactions to the Total Synthesis of Phorboxazole B
J. Am. Chem. Soc. 2000, 122, 10033-10046 10033 Application of Complex Aldol Reactions to the Total Synthesis of Phorboxazole B David A. Evans,* Duke M. Fitch,1 Thomas E. Smith, and Victor J. Cee Contribution from the Department of Chemistry and Chemical Biology, HarVard UniVersity, Cambridge, Massachusetts 02138 ReceiVed June 29, 2000 Abstract: The synthesis of phorboxazole B has been accomplished in 27 linear steps and an overall yield of 12.6%. The absolute stereochemistry of the C4-C12,C33-C38, and C13-C19 fragments was established utilizing catalytic asymmetric aldol methodology, while the absolute stereochemistry of the C20-C32 fragment was derived from an auxiliary-based asymmetric aldol reaction. All remaining chirality was incorporated through internal asymmetric induction, with the exception of the C43 stereocenter which was derived from (R)-trityl glycidol. Key fragment couplings include a stereoselective double stereodifferentiating aldol reaction, a metalated oxazole alkylation, an oxazole-stabilized Wittig olefination, and a chelation-controlled addition of the fully elaborated alkenyl metal side chain. Introduction and HT29 (3.31 × 10-10 M), as well as leukemia CCRF-CBM (2.45 × 10-10 M), prostate cancer PC-3 (3.54 × 10-10 M), and Phorboxazoles A (1)andB(2) are marine natural products breast cancer MCF7 cell lines (5.62 × 10-10 M).2b In addition isolated from a recently discovered species of Indian Ocean to this impressive anticancer activity, phorboxazoles A and B sponge (genus Phorbas sp.) near Muiron Island, Western also exhibit potent in vitro antifungal activity against Candida Australia.2 These substances are representative of a new class albicans and Saccharomyces carlsbergensis at 0.1 µg/disk in of macrolides differing only in their C hydroxyl-bearing 13 the agar disk diffusion assay.2a stereocenters.