Substantial Equivalence Determination Decision Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vaccinia Belongs to a Family of Viruses That Is Closely Related to the Smallpox Virus
VACCINIA INFECTION What is it? Vaccinia belongs to a family of viruses that is closely related to the smallpox virus. Because of the similarities between the smallpox and vaccinia viruses, the vaccinia virus is used in the smallpox vaccine. When this virus is used as a vaccine, it allows our immune systems to develop immunity against smallpox. The smallpox vaccine does not actually contain smallpox virus and cannot cause smallpox. Vaccination usually prevents smallpox infection for at least ten years. The vaccinia vaccine against smallpox was used to successfully eradicate smallpox from the human population. More recently, this virus has also become of interest due to concerns about smallpox being used as an agent of bioterrorism. How is the virus spread? Vaccinia can be spread by touching the vaccination site before it has fully healed or by touching clothing or bandages that have been contaminated with the live virus during vaccination. In this manner, vaccinia can spread to other parts of the body and to other individuals. It cannot be spread through the air. What are the symptoms of vaccinia? Vaccinia virus symptoms are similar to smallpox, but milder. Vaccinia may cause rash, fever, headache and body aches. In certain individuals, such as those with weak immune systems, the symptoms can be more severe. What are the potential side effects of the vaccinia vaccine for smallpox? Normal reactions are mild and go away without any treatment.These include: Soreness and redness in the arm where the vaccine was given Slightly swollen, sore glands in the armpits Low grade fever One in approximately three people will feel badly enough to miss school, work or recreational activities Trouble sleeping Serious reactions are not very common but can occur in about 1,000 in every 1 million people who are vaccinated for the first time. -

The Munich Outbreak of Cutaneous Cowpox Infection: Transmission by Infected Pet Rats
Acta Derm Venereol 2012; 92: 126–131 INVESTIGATIVE REPORT The Munich Outbreak of Cutaneous Cowpox Infection: Transmission by Infected Pet Rats Sandra VOGEL1, Miklós SÁRDY1, Katharina GLOS2, Hans Christian KOrting1, Thomas RUZICKA1 and Andreas WOLLENBERG1 1Department of Dermatology and Allergology, Ludwig Maximilian University, Munich, and 2Department of Dermatology, Haas and Link Animal Clinic, Germering, Germany Cowpox virus infection of humans is an uncommon, another type of orthopoxvirus, from infected pet prairie potentially fatal, skin disease. It is largely confined to dogs have recently been described in the USA, making Europe, but is not found in Eire, or in the USA, Austral the medical community aware of the risk of transmission asia, or the Middle or Far East. Patients having contact of pox viruses from pets (3). with infected cows, cats, or small rodents sporadically Seven of 8 exposed patients living in the Munich contract the disease from these animals. We report here area contracted cowpox virus infection from an unusual clinical aspects of 8 patients from the Munich area who source: rats infected with cowpox virus bought from had purchased infected pet rats from a local supplier. Pet local pet shops and reputedly from the same supplier rats are a novel potential source of local outbreaks. The caused a clinically distinctive pattern of infection, which morphologically distinctive skin lesions are mostly res was mostly restricted to the patients’ neck and trunk. tricted to the patients’ necks, reflecting the infected ani We report here dermatologically relevant aspects of mals’ contact pattern. Individual lesions vaguely resem our patients in order to alert the medical community to ble orf or Milker’s nodule, but show marked surrounding the possible risk of a zoonotic orthopoxvirus outbreak erythema, firm induration and local adenopathy. -

Vaccinia Viruses As Vectors for Vaccine Antigens Vaccinia Viruses As Vectors for Vaccine Antigens
Vaccinia Viruses as Vectors for Vaccine Antigens Vaccinia Viruses as Vectors for Vaccine Antigens Proceedings of the Workshop on Vaccinia Viruses as Vectors for Vaccine Antigens, held November 13-14, 1984, in Chevy Chase, Maryland, U. S.A. Editor: GeraldV.Quinnan,Jr.,M.D. Director, Division of Virology Center for Drugs and Biologics Food and Drug Administration Bethesda, Maryland Elsevier New York • Amsterdam. Oxford .... i © 1985 by Elsevier Science Publishing Co., Inc. All rights reserved. This book has been registered with the Copyright Clearance Center, Inc. For further information, please contact the Copyright Clearance Center, Salem, Massachusetts. Published by: Elsevier Science Publishing Co., Inc. 52 Vanderbilt Avenue, New York, New York 10017 Sole distributors outside the United States and Canada: Elsevier Science Publishers B.V. P.O. Box 211, 1000 AE Amsterdam, the Netherlands Library of Congress Cataloging in Publication Data Workshop in Vaccinia Viruses as Vectors for Vaccine Antigens (1984: Chevy Chase, Md.) Vaccinia viruses as vectors for vaccine antigens. Includes index. I. Vaccines--Congresses. 2. Vaccinia--Congresses. 3. Viralantigens-- Congresses. 4. Smallpox--Congresses. I. Quinnan, Gerald V. 1I. Title. [DNLM: 1. Antigens, Viral--immunology--Congresses. 2. VacciniaVirus-- genetics--congresses. 3. VacciniaVirus--immunology--congresses. 4. Viral Vaccines--immunology--congresses. QW 165.5.P6 W926v 1984] QR189.W674 1984 615'.372 85-12960 ISBN 0-444-00984-1 Manufactured in the United States of America CONTENTS Preface ..... ..... ° . ............... ix Participants .............................. xi Part I: BIOLOGY OF VACCINIA AND OTHER ORTHOPOX VIRUSES I Chairpersons: G. Schild and J. Nakano Vaccinia Virus ...................... 3 D. Baxby Aspects of the Biology of Orthopox Viruses Relevant to the Use of Recombinant Vaccines as Field Vaccines ..... -

Where Do We Stand After Decades of Studying Human Cytomegalovirus?
microorganisms Review Where do we Stand after Decades of Studying Human Cytomegalovirus? 1, 2, 1 1 Francesca Gugliesi y, Alessandra Coscia y, Gloria Griffante , Ganna Galitska , Selina Pasquero 1, Camilla Albano 1 and Matteo Biolatti 1,* 1 Laboratory of Pathogenesis of Viral Infections, Department of Public Health and Pediatric Sciences, University of Turin, 10126 Turin, Italy; [email protected] (F.G.); gloria.griff[email protected] (G.G.); [email protected] (G.G.); [email protected] (S.P.); [email protected] (C.A.) 2 Complex Structure Neonatology Unit, Department of Public Health and Pediatric Sciences, University of Turin, 10126 Turin, Italy; [email protected] * Correspondence: [email protected] These authors contributed equally to this work. y Received: 19 March 2020; Accepted: 5 May 2020; Published: 8 May 2020 Abstract: Human cytomegalovirus (HCMV), a linear double-stranded DNA betaherpesvirus belonging to the family of Herpesviridae, is characterized by widespread seroprevalence, ranging between 56% and 94%, strictly dependent on the socioeconomic background of the country being considered. Typically, HCMV causes asymptomatic infection in the immunocompetent population, while in immunocompromised individuals or when transmitted vertically from the mother to the fetus it leads to systemic disease with severe complications and high mortality rate. Following primary infection, HCMV establishes a state of latency primarily in myeloid cells, from which it can be reactivated by various inflammatory stimuli. Several studies have shown that HCMV, despite being a DNA virus, is highly prone to genetic variability that strongly influences its replication and dissemination rates as well as cellular tropism. In this scenario, the few currently available drugs for the treatment of HCMV infections are characterized by high toxicity, poor oral bioavailability, and emerging resistance. -

Characterization of Host Micrornas That Respond to DNA Virus Infection in a Crustacean Tianzhi Huang, Dandan Xu and Xiaobo Zhang*
Huang et al. BMC Genomics 2012, 13:159 http://www.biomedcentral.com/1471-2164/13/159 RESEARCH ARTICLE Open Access Characterization of host microRNAs that respond to DNA virus infection in a crustacean Tianzhi Huang, Dandan Xu and Xiaobo Zhang* Abstract Background: MicroRNAs (miRNAs) are key posttranscriptional regulators of gene expression that are implicated in many processes of eukaryotic cells. It is known that the expression profiles of host miRNAs can be reshaped by viruses. However, a systematic investigation of marine invertebrate miRNAs that respond to virus infection has not yet been performed. Results: In this study, the shrimp Marsupenaeus japonicus was challenged by white spot syndrome virus (WSSV). Small RNA sequencing of WSSV-infected shrimp at different time post-infection (0, 6, 24 and 48 h) identified 63 host miRNAs, 48 of which were conserved in other animals, representing 43 distinct families. Of the identified host miRNAs, 31 were differentially expressed in response to virus infection, of which 25 were up-regulated and six down-regulated. The results were confirmed by northern blots. The TargetScan and miRanda algorithms showed that most target genes of the differentially expressed miRNAs were related to immune responses. Gene ontology analysis revealed that immune signaling pathways were mediated by these miRNAs. Evolutionary analysis showed that three of them, miR-1, miR-7 and miR-34, are highly conserved in shrimp, fruit fly and humans and function in the similar pathways. Conclusions: Our study provides the first large-scale characterization of marine invertebrate miRNAs that respond to virus infection. This will help to reveal the molecular events involved in virus-host interactions mediated by miRNAs and their evolution in animals. -

Vaccinia Virus
APPENDIX 2 Vaccinia Virus • Accidental infection following transfer from the vac- cination site to another site (autoinoculation) or to Disease Agent: another person following intimate contact Likelihood of Secondary Transmission: • Vaccinia virus • Significant following direct contact Disease Agent Characteristics: At-Risk Populations: • Family: Poxviridae; Subfamily: Chordopoxvirinae; • Individuals receiving smallpox (vaccinia) vaccination Genus: Orthopoxvirus • Individuals who come in direct contact with vacci- • Virion morphology and size: Enveloped, biconcave nated persons core with two lateral bodies, brick-shaped to pleo- • Those at risk for more severe complications of infec- morphic virions, ~360 ¥ 270 ¥ 250 nm in size tion include the following: • Nucleic acid: Nonsegmented, linear, covalently ᭺ Immune-compromised persons including preg- closed, double-stranded DNA, 18.9-20.0 kb in length nant women • Physicochemical properties: Virus is inactivated at ᭺ Patients with atopy, especially those with eczema 60°C for 8 minutes, but antigen can withstand 100°C; ᭺ Patients with extensive exfoliative skin disease lyophilized virus maintains potency for 18 months at 4-6°C; virus may be stable when dried onto inanimate Vector and Reservoir Involved: surfaces; susceptible to 1% sodium hypochlorite, • No natural host 2% glutaraldehyde, and formaldehyde; disinfection of hands and environmental contamination with soap Blood Phase: and water are effective • Vaccinia DNA was detected by PCR in the blood in 6.5% of 77 military members from 1 to 3 weeks after Disease Name: smallpox (vaccinia) vaccination that resulted in a major skin reaction. • Progressive vaccinia (vaccinia necrosum or vaccinia • In the absence of complications after immunization, gangrenosum) recently published PCR and culture data suggest that • Generalized vaccinia viremia with current vaccines must be rare 3 weeks • Eczema vaccinatum after vaccination. -

Specimen Type, Collection Methods, and Diagnostic Assays Available For
Specimen type, collection methods, and diagnostic assays available for the detection of poxviruses from human specimens by the Poxvirus and Rabies Branch, Centers for Disease Control and Prevention1. Specimen Orthopoxvirus Parapoxvirus Yatapoxvirus Molluscipoxvirus Specimen type collection method PCR6 Culture EM8 IHC9,10 Serology11 PCR12 EM8 IHC9,10 PCR13 EM8 PCR EM8 Lesion material Fresh or frozen Swab 5 Lesion material [dry or in media ] [vesicle / pustule Formalin fixed skin, scab / crust, etc.] Paraffin block Fixed slide(s) Container Lesion fluid Swab [vesicle / pustule [dry or in media5] fluid, etc.] Touch prep slide Blood EDTA2 EDTA tube 7 Spun or aliquoted Serum before shipment Spun or aliquoted Plasma before shipment CSF3,4 Sterile 1. The detection of poxviruses by electron microscopy (EM) and immunohistochemical staining (IHC) is performed by the Infectious Disease Pathology Branch of the CDC. 2. EDTA — Ethylenediaminetetraacetic acid. 3. CSF — Cerebrospinal fluid. 4. In order to accurately interpret test results generated from CSF specimens, paired serum must also be submitted. 5. If media is used to store and transport specimens a minimal amount should be used to ensure as little dilution of DNA as possible. 6. Orthopoxvirus generic real-time polymerase chain reaction (PCR) assays will amplify DNA from numerous species of virus within the Orthopoxvirus genus. Species-specific real-time PCR assays are available for selective detection of DNA from variola virus, vaccinia virus, monkeypox virus, and cowpox virus. 7. Blood is not ideal for the detection of orthopoxviruses by PCR as the period of viremia has often passed before sampling occurs. 8. EM can reveal the presence of a poxvirus in clinical specimens or from virus culture, but this technique cannot differentiate between virus species within the same genus. -
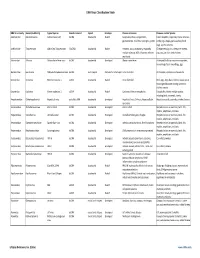
Virus Classification Tables V2.Vd.Xlsx
DNA Virus Classification Table DNA Virus Family Genera (Subfamily) Typical Species Genetic material Capsid Envelope Disease in Humans Diseases in other Species Adenoviridae Mastadenovirus Adenoviruses 1‐47 dsDNA Icosahedral Naked Respiratory illness; conjunctivitis, Canine hepatitis, respiratory illness in horses, gastroenteritis, tonsillitis, meningitis, cystitis cattle, pigs, sheep, goats, sea lions, birds dogs, squirrel enteritis Anelloviridae Torqueviruses Alpha‐Zeta Torqueviruses (‐)ssDNA Icosahedral Naked Hepatitis, lupus, pulmonary, myopathy, Chimpanzee, pig, cow, sheep, tree shrews, multiple sclerosis; 90% of humans infected pigs, cats, sea lions and chickens worldwide Asfarviridae Asfivirus African Swine fever virus dsDNA Icosahedral Enveloped African swine fever Arthropod (tick) transmission or ingestion; hemorrhagic fever in warthogs, pigs Baculoviridae Baculovirus Alpha‐Gamma Baculoviruses dsDNA Stick shaped Occluded or Enveloped none identified Arthropods, Lepidoptera, crustaceans Circoviridae Circovirus Porcine circovirus 1 ssDNA Icosahedral Naked none identified Birds, pigs, dogs; bats; rodents; causes post‐ weaning multisystem wasting syndrome, chicken anemia Circoviridae Cyclovirus Human cyclovirus 1 ssDNA Icosahedral Naked Cyclovirus Vietnam encephalitis Encephalitis; infects multiple species including birds, mammals, insects Hepadnaviridae Orthohepadnavirus Hepatitis B virus partially ssDNA Icosahedral Enveloped Hepatitis B virus; Cirrhosis, Hepatocellular Hepatitis in ducks, squirrels, primates, herons carcinoma Herpesviridae -

Evaluation of Recombinant Vaccinia Virus—Measles Vaccines in Infant Rhesus Macaques with Preexisting Measles Antibody
Virology 276, 202–213 (2000) doi:10.1006/viro.2000.0564, available online at http://www.idealibrary.com on Evaluation of Recombinant Vaccinia Virus—Measles Vaccines in Infant Rhesus Macaques with Preexisting Measles Antibody Yong-de Zhu,*,1 Paul Rota,† Linda Wyatt,‡ Azaibi Tamin,† Shmuel Rozenblatt,‡,2 Nicholas Lerche,* Bernard Moss,‡ William Bellini,† and Michael McChesney*,§,3 *The California Regional Primate Research Center and §Department of Pathology, School of Medicine, University of California, Davis, California 95616; †Measles Virus Section, Respiratory and Enteric Viruses Branch, Division of Viral and Rickettsial Diseases, National Center for Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia 30333; and ‡Laboratory of Viral Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland 20892 Received June 20, 2000; returned to author for revision July 28, 2000; accepted August 1, 2000 Immunization of newborn infants with standard measles vaccines is not effective because of the presence of maternal antibody. In this study, newborn rhesus macaques were immunized with recombinant vaccinia viruses expressing measles virus hemagglutinin (H) and fusion (F) proteins, using the replication-competent WR strain of vaccinia virus or the replication- defective MVA strain. The infants were boosted at 2 months and then challenged intranasally with measles virus at 5 months of age. Some of the newborn monkeys received measles immune globulin (MIG) prior to the first immunization, and these infants were compared to additional infants that had maternal measles-neutralizing antibody. In the absence of measles antibody, vaccination with either vector induced neutralizing antibody, cytotoxic T cell (CTL) responses to measles virus and protection from systemic measles infection and skin rash. -

Monkeypox Virus Emergence in Wild Chimpanzees Reveals Distinct Clinical Outcomes and Viral Diversity
ARTICLES https://doi.org/10.1038/s41564-020-0706-0 Monkeypox virus emergence in wild chimpanzees reveals distinct clinical outcomes and viral diversity Livia V. Patrono1,6, Kamilla Pléh1,2,6, Liran Samuni2,3, Markus Ulrich1, Caroline Röthemeier1, Andreas Sachse1, Silvia Muschter4, Andreas Nitsche4, Emmanuel Couacy-Hymann5, Christophe Boesch2,3, Roman M. Wittig 2,3, Sébastien Calvignac-Spencer1 and Fabian H. Leendertz 1 ✉ Monkeypox is a viral zoonotic disease on the rise across endemic habitats. Despite the growing importance of monkeypox virus, our knowledge on its host spectrum and sylvatic maintenance is limited. Here, we describe the recent repeated emergence of monkeypox virus in a wild, human-habituated western chimpanzee (Pan troglodytes verus, hereafter chimpanzee) population from Taï National Park, Ivory Coast. Through daily monitoring, we show that further to causing its typical exanthematous syn- drome, monkeypox can present itself as a severe respiratory disease without a diffuse rash. By analysing 949 non-invasively collected samples, we identify the circulation of at least two distinct monkeypox virus lineages and document the shedding of infectious particles in faeces and flies, suggesting that they could mediate indirect transmission. We also show that the carnivo- rous component of the Taï chimpanzees’ diet, mainly consisting of the sympatric monkeys they regularly hunt, did not change nor shift towards rodent consumption (the presumed reservoir) before the outbreaks, suggesting that the sudden emergence of monkeypox virus in this population is probably due to changes in the ecology of the virus itself. Using long-term mortality surveillance data from Taï National Park, we provide evidence of little to no prior viral activity over at least two decades. -

Effects of Pre-Existing Orthopoxvirus-Specific Immunity On
www.nature.com/scientificreports OPEN Efects of pre-existing orthopoxvirus-specifc immunity on the performance of Modifed Received: 4 January 2018 Accepted: 10 April 2018 Vaccinia virus Ankara-based Published: xx xx xxxx infuenza vaccines Arwen F. Altenburg1, Stella E. van Trierum1, Erwin de Bruin1, Dennis de Meulder1, Carolien E. van de Sandt1, Fiona R. M. van der Klis2, Ron A. M. Fouchier1, Marion P. G. Koopmans1, Guus F. Rimmelzwaan1,3 & Rory D. de Vries1 The replication-defcient orthopoxvirus modifed vaccinia virus Ankara (MVA) is a promising vaccine vector against various pathogens and has an excellent safety record. However, pre-existing vector- specifc immunity is frequently suggested to be a drawback of MVA-based vaccines. To address this issue, mice were vaccinated with MVA-based infuenza vaccines in the presence or absence of orthopoxvirus-specifc immunity. Importantly, protective efcacy of an MVA-based infuenza vaccine against a homologous challenge was not impaired in the presence of orthopoxvirus-specifc pre-existing immunity. Nonetheless, orthopoxvirus-specifc pre-existing immunity reduced the induction of antigen- specifc antibodies under specifc conditions and completely prevented induction of antigen-specifc T cell responses by rMVA-based vaccination. Notably, antibodies induced by vaccinia virus vaccination, both in mice and humans, were not capable of neutralizing MVA. Thus, when using rMVA-based vaccines it is important to consider the main correlate of protection induced by the vaccine, the vaccine dose and the orthopoxvirus immune status of vaccine recipients. Recombinant viral vectors are under development as novel vaccine candidates that induce immunity to antigens of interest expressed from transgenes. -

Risk Groups: Viruses (C) 1988, American Biological Safety Association
Rev.: 1.0 Risk Groups: Viruses (c) 1988, American Biological Safety Association BL RG RG RG RG RG LCDC-96 Belgium-97 ID Name Viral group Comments BMBL-93 CDC NIH rDNA-97 EU-96 Australia-95 HP AP (Canada) Annex VIII Flaviviridae/ Flavivirus (Grp 2 Absettarov, TBE 4 4 4 implied 3 3 4 + B Arbovirus) Acute haemorrhagic taxonomy 2, Enterovirus 3 conjunctivitis virus Picornaviridae 2 + different 70 (AHC) Adenovirus 4 Adenoviridae 2 2 (incl animal) 2 2 + (human,all types) 5 Aino X-Arboviruses 6 Akabane X-Arboviruses 7 Alastrim Poxviridae Restricted 4 4, Foot-and- 8 Aphthovirus Picornaviridae 2 mouth disease + viruses 9 Araguari X-Arboviruses (feces of children 10 Astroviridae Astroviridae 2 2 + + and lambs) Avian leukosis virus 11 Viral vector/Animal retrovirus 1 3 (wild strain) + (ALV) 3, (Rous 12 Avian sarcoma virus Viral vector/Animal retrovirus 1 sarcoma virus, + RSV wild strain) 13 Baculovirus Viral vector/Animal virus 1 + Togaviridae/ Alphavirus (Grp 14 Barmah Forest 2 A Arbovirus) 15 Batama X-Arboviruses 16 Batken X-Arboviruses Togaviridae/ Alphavirus (Grp 17 Bebaru virus 2 2 2 2 + A Arbovirus) 18 Bhanja X-Arboviruses 19 Bimbo X-Arboviruses Blood-borne hepatitis 20 viruses not yet Unclassified viruses 2 implied 2 implied 3 (**)D 3 + identified 21 Bluetongue X-Arboviruses 22 Bobaya X-Arboviruses 23 Bobia X-Arboviruses Bovine 24 immunodeficiency Viral vector/Animal retrovirus 3 (wild strain) + virus (BIV) 3, Bovine Bovine leukemia 25 Viral vector/Animal retrovirus 1 lymphosarcoma + virus (BLV) virus wild strain Bovine papilloma Papovavirus/