Characterization of Host Micrornas That Respond to DNA Virus Infection in a Crustacean Tianzhi Huang, Dandan Xu and Xiaobo Zhang*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Novel 2-Herpesvirus of the Rhadinovirus 2 Lineage In
Downloaded from genome.cshlp.org on September 29, 2021 - Published by Cold Spring Harbor Laboratory Press Letter A Novel ␥2-Herpesvirus of the Rhadinovirus 2 Lineage in Chimpanzees Vincent Lacoste,1 Philippe Mauclère,1,2 Guy Dubreuil,3 John Lewis,4 Marie-Claude Georges-Courbot,3 andAntoine Gessain 1,5 1Unite´d’Epide´miologie et Physiopathologie des Virus Oncoge`nes, De´partement du SIDA et des Re´trovirus, Institut Pasteur, 75724 Paris Cedex 15, France; 2Centre Pasteur du Cameroun, BP 1274, Yaounde´, Cameroon; 3Centre International de Recherches Me´dicales, Franceville, Gabon; 4International Zoo Veterinary Group, Keighley, West Yorkshire BD21 1AG, UK Old World monkeys and, recently, African great apes have been shown, by serology and polymerase chain reaction (PCR), to harbor different ␥2-herpesviruses closely related to Kaposi’s sarcoma-associated Herpesvirus (KSHV). Although the presence of two distinct lineages of KSHV-like rhadinoviruses, RV1 and RV2, has been revealed in Old World primates (including African green monkeys, macaques, and, recently, mandrills), viruses belonging to the RV2 genogroup have not yet been identified from great apes. Indeed, the three yet known ␥2-herpesviruses in chimpanzees (PanRHV1a/PtRV1, PanRHV1b) and gorillas (GorRHV1) belong to the RV1 group. To investigate the putative existence of a new RV2 Rhadinovirus in chimpanzees and gorillas we have used the degenerate consensus primer PCR strategy for the Herpesviral DNA polymerase gene on 40 wild-caught animals. This study led to the discovery, in common chimpanzees, of a novel ␥2-herpesvirus belonging to the RV2 genogroup, termed Pan Rhadino-herpesvirus 2 (PanRHV2). Use of specific primers and internal oligonucleotide probes demonstrated the presence of this novel ␥2-herpesvirus in three wild-caught animals. -

The Munich Outbreak of Cutaneous Cowpox Infection: Transmission by Infected Pet Rats
Acta Derm Venereol 2012; 92: 126–131 INVESTIGATIVE REPORT The Munich Outbreak of Cutaneous Cowpox Infection: Transmission by Infected Pet Rats Sandra VOGEL1, Miklós SÁRDY1, Katharina GLOS2, Hans Christian KOrting1, Thomas RUZICKA1 and Andreas WOLLENBERG1 1Department of Dermatology and Allergology, Ludwig Maximilian University, Munich, and 2Department of Dermatology, Haas and Link Animal Clinic, Germering, Germany Cowpox virus infection of humans is an uncommon, another type of orthopoxvirus, from infected pet prairie potentially fatal, skin disease. It is largely confined to dogs have recently been described in the USA, making Europe, but is not found in Eire, or in the USA, Austral the medical community aware of the risk of transmission asia, or the Middle or Far East. Patients having contact of pox viruses from pets (3). with infected cows, cats, or small rodents sporadically Seven of 8 exposed patients living in the Munich contract the disease from these animals. We report here area contracted cowpox virus infection from an unusual clinical aspects of 8 patients from the Munich area who source: rats infected with cowpox virus bought from had purchased infected pet rats from a local supplier. Pet local pet shops and reputedly from the same supplier rats are a novel potential source of local outbreaks. The caused a clinically distinctive pattern of infection, which morphologically distinctive skin lesions are mostly res was mostly restricted to the patients’ neck and trunk. tricted to the patients’ necks, reflecting the infected ani We report here dermatologically relevant aspects of mals’ contact pattern. Individual lesions vaguely resem our patients in order to alert the medical community to ble orf or Milker’s nodule, but show marked surrounding the possible risk of a zoonotic orthopoxvirus outbreak erythema, firm induration and local adenopathy. -

Journal of Virology
JOURNAL OF VIROLOGY Volume 80 March 2006 No. 6 SPOTLIGHT Articles of Significant Interest Selected from This Issue by 2587–2588 the Editors STRUCTURE AND ASSEMBLY Crystal Structure of the Oligomerization Domain of the Haitao Ding, Todd J. Green, 2808–2814 Phosphoprotein of Vesicular Stomatitis Virus Shanyun Lu, and Ming Luo Subcellular Localization of Hepatitis C Virus Structural Yves Rouille´, Franc¸ois Helle, David 2832–2841 Proteins in a Cell Culture System That Efficiently Replicates Delgrange, Philippe Roingeard, the Virus Ce´cile Voisset, Emmanuelle Blanchard, Sandrine Belouzard, Jane McKeating, Arvind H. Patel, Geert Maertens, Takaji Wakita, Czeslaw Wychowski, and Jean Dubuisson A Small Loop in the Capsid Protein of Moloney Murine Marcy R. Auerbach, Kristy R. 2884–2893 Leukemia Virus Controls Assembly of Spherical Cores Brown, Artem Kaplan, Denise de Las Nueces, and Ila R. Singh Identification of the Nucleocapsid, Tegument, and Envelope Jyh-Ming Tsai, Han-Ching Wang, 3021–3029 Proteins of the Shrimp White Spot Syndrome Virus Virion Jiann-Horng Leu, Andrew H.-J. Wang, Ying Zhuang, Peter J. Walker, Guang-Hsiung Kou, and Chu-Fang Lo GENOME REPLICATION AND REGULATION OF VIRAL GENE EXPRESSION Epitope Mapping of Herpes Simplex Virus Type 2 gH/gL Tina M. Cairns, Marie S. Shaner, Yi 2596–2608 Defines Distinct Antigenic Sites, Including Some Associated Zuo, Manuel Ponce-de-Leon, with Biological Function Isabelle Baribaud, Roselyn J. Eisenberg, Gary H. Cohen, and J. Charles Whitbeck The ␣-TIF (VP16) Homologue (ETIF) of Equine Jens von Einem, Daniel 2609–2620 Herpesvirus 1 Is Essential for Secondary Envelopment Schumacher, Dennis J. O’Callaghan, and Virus Egress and Nikolaus Osterrieder Suppression of Viral RNA Recombination by a Host Chi-Ping Cheng, Elena Serviene, 2631–2640 Exoribonuclease and Peter D. -

Where Do We Stand After Decades of Studying Human Cytomegalovirus?
microorganisms Review Where do we Stand after Decades of Studying Human Cytomegalovirus? 1, 2, 1 1 Francesca Gugliesi y, Alessandra Coscia y, Gloria Griffante , Ganna Galitska , Selina Pasquero 1, Camilla Albano 1 and Matteo Biolatti 1,* 1 Laboratory of Pathogenesis of Viral Infections, Department of Public Health and Pediatric Sciences, University of Turin, 10126 Turin, Italy; [email protected] (F.G.); gloria.griff[email protected] (G.G.); [email protected] (G.G.); [email protected] (S.P.); [email protected] (C.A.) 2 Complex Structure Neonatology Unit, Department of Public Health and Pediatric Sciences, University of Turin, 10126 Turin, Italy; [email protected] * Correspondence: [email protected] These authors contributed equally to this work. y Received: 19 March 2020; Accepted: 5 May 2020; Published: 8 May 2020 Abstract: Human cytomegalovirus (HCMV), a linear double-stranded DNA betaherpesvirus belonging to the family of Herpesviridae, is characterized by widespread seroprevalence, ranging between 56% and 94%, strictly dependent on the socioeconomic background of the country being considered. Typically, HCMV causes asymptomatic infection in the immunocompetent population, while in immunocompromised individuals or when transmitted vertically from the mother to the fetus it leads to systemic disease with severe complications and high mortality rate. Following primary infection, HCMV establishes a state of latency primarily in myeloid cells, from which it can be reactivated by various inflammatory stimuli. Several studies have shown that HCMV, despite being a DNA virus, is highly prone to genetic variability that strongly influences its replication and dissemination rates as well as cellular tropism. In this scenario, the few currently available drugs for the treatment of HCMV infections are characterized by high toxicity, poor oral bioavailability, and emerging resistance. -

Specimen Type, Collection Methods, and Diagnostic Assays Available For
Specimen type, collection methods, and diagnostic assays available for the detection of poxviruses from human specimens by the Poxvirus and Rabies Branch, Centers for Disease Control and Prevention1. Specimen Orthopoxvirus Parapoxvirus Yatapoxvirus Molluscipoxvirus Specimen type collection method PCR6 Culture EM8 IHC9,10 Serology11 PCR12 EM8 IHC9,10 PCR13 EM8 PCR EM8 Lesion material Fresh or frozen Swab 5 Lesion material [dry or in media ] [vesicle / pustule Formalin fixed skin, scab / crust, etc.] Paraffin block Fixed slide(s) Container Lesion fluid Swab [vesicle / pustule [dry or in media5] fluid, etc.] Touch prep slide Blood EDTA2 EDTA tube 7 Spun or aliquoted Serum before shipment Spun or aliquoted Plasma before shipment CSF3,4 Sterile 1. The detection of poxviruses by electron microscopy (EM) and immunohistochemical staining (IHC) is performed by the Infectious Disease Pathology Branch of the CDC. 2. EDTA — Ethylenediaminetetraacetic acid. 3. CSF — Cerebrospinal fluid. 4. In order to accurately interpret test results generated from CSF specimens, paired serum must also be submitted. 5. If media is used to store and transport specimens a minimal amount should be used to ensure as little dilution of DNA as possible. 6. Orthopoxvirus generic real-time polymerase chain reaction (PCR) assays will amplify DNA from numerous species of virus within the Orthopoxvirus genus. Species-specific real-time PCR assays are available for selective detection of DNA from variola virus, vaccinia virus, monkeypox virus, and cowpox virus. 7. Blood is not ideal for the detection of orthopoxviruses by PCR as the period of viremia has often passed before sampling occurs. 8. EM can reveal the presence of a poxvirus in clinical specimens or from virus culture, but this technique cannot differentiate between virus species within the same genus. -
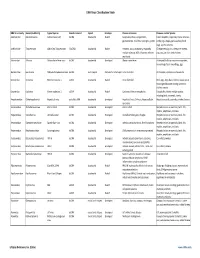
Virus Classification Tables V2.Vd.Xlsx
DNA Virus Classification Table DNA Virus Family Genera (Subfamily) Typical Species Genetic material Capsid Envelope Disease in Humans Diseases in other Species Adenoviridae Mastadenovirus Adenoviruses 1‐47 dsDNA Icosahedral Naked Respiratory illness; conjunctivitis, Canine hepatitis, respiratory illness in horses, gastroenteritis, tonsillitis, meningitis, cystitis cattle, pigs, sheep, goats, sea lions, birds dogs, squirrel enteritis Anelloviridae Torqueviruses Alpha‐Zeta Torqueviruses (‐)ssDNA Icosahedral Naked Hepatitis, lupus, pulmonary, myopathy, Chimpanzee, pig, cow, sheep, tree shrews, multiple sclerosis; 90% of humans infected pigs, cats, sea lions and chickens worldwide Asfarviridae Asfivirus African Swine fever virus dsDNA Icosahedral Enveloped African swine fever Arthropod (tick) transmission or ingestion; hemorrhagic fever in warthogs, pigs Baculoviridae Baculovirus Alpha‐Gamma Baculoviruses dsDNA Stick shaped Occluded or Enveloped none identified Arthropods, Lepidoptera, crustaceans Circoviridae Circovirus Porcine circovirus 1 ssDNA Icosahedral Naked none identified Birds, pigs, dogs; bats; rodents; causes post‐ weaning multisystem wasting syndrome, chicken anemia Circoviridae Cyclovirus Human cyclovirus 1 ssDNA Icosahedral Naked Cyclovirus Vietnam encephalitis Encephalitis; infects multiple species including birds, mammals, insects Hepadnaviridae Orthohepadnavirus Hepatitis B virus partially ssDNA Icosahedral Enveloped Hepatitis B virus; Cirrhosis, Hepatocellular Hepatitis in ducks, squirrels, primates, herons carcinoma Herpesviridae -

Monkeypox Virus Emergence in Wild Chimpanzees Reveals Distinct Clinical Outcomes and Viral Diversity
ARTICLES https://doi.org/10.1038/s41564-020-0706-0 Monkeypox virus emergence in wild chimpanzees reveals distinct clinical outcomes and viral diversity Livia V. Patrono1,6, Kamilla Pléh1,2,6, Liran Samuni2,3, Markus Ulrich1, Caroline Röthemeier1, Andreas Sachse1, Silvia Muschter4, Andreas Nitsche4, Emmanuel Couacy-Hymann5, Christophe Boesch2,3, Roman M. Wittig 2,3, Sébastien Calvignac-Spencer1 and Fabian H. Leendertz 1 ✉ Monkeypox is a viral zoonotic disease on the rise across endemic habitats. Despite the growing importance of monkeypox virus, our knowledge on its host spectrum and sylvatic maintenance is limited. Here, we describe the recent repeated emergence of monkeypox virus in a wild, human-habituated western chimpanzee (Pan troglodytes verus, hereafter chimpanzee) population from Taï National Park, Ivory Coast. Through daily monitoring, we show that further to causing its typical exanthematous syn- drome, monkeypox can present itself as a severe respiratory disease without a diffuse rash. By analysing 949 non-invasively collected samples, we identify the circulation of at least two distinct monkeypox virus lineages and document the shedding of infectious particles in faeces and flies, suggesting that they could mediate indirect transmission. We also show that the carnivo- rous component of the Taï chimpanzees’ diet, mainly consisting of the sympatric monkeys they regularly hunt, did not change nor shift towards rodent consumption (the presumed reservoir) before the outbreaks, suggesting that the sudden emergence of monkeypox virus in this population is probably due to changes in the ecology of the virus itself. Using long-term mortality surveillance data from Taï National Park, we provide evidence of little to no prior viral activity over at least two decades. -

Effects of Pre-Existing Orthopoxvirus-Specific Immunity On
www.nature.com/scientificreports OPEN Efects of pre-existing orthopoxvirus-specifc immunity on the performance of Modifed Received: 4 January 2018 Accepted: 10 April 2018 Vaccinia virus Ankara-based Published: xx xx xxxx infuenza vaccines Arwen F. Altenburg1, Stella E. van Trierum1, Erwin de Bruin1, Dennis de Meulder1, Carolien E. van de Sandt1, Fiona R. M. van der Klis2, Ron A. M. Fouchier1, Marion P. G. Koopmans1, Guus F. Rimmelzwaan1,3 & Rory D. de Vries1 The replication-defcient orthopoxvirus modifed vaccinia virus Ankara (MVA) is a promising vaccine vector against various pathogens and has an excellent safety record. However, pre-existing vector- specifc immunity is frequently suggested to be a drawback of MVA-based vaccines. To address this issue, mice were vaccinated with MVA-based infuenza vaccines in the presence or absence of orthopoxvirus-specifc immunity. Importantly, protective efcacy of an MVA-based infuenza vaccine against a homologous challenge was not impaired in the presence of orthopoxvirus-specifc pre-existing immunity. Nonetheless, orthopoxvirus-specifc pre-existing immunity reduced the induction of antigen- specifc antibodies under specifc conditions and completely prevented induction of antigen-specifc T cell responses by rMVA-based vaccination. Notably, antibodies induced by vaccinia virus vaccination, both in mice and humans, were not capable of neutralizing MVA. Thus, when using rMVA-based vaccines it is important to consider the main correlate of protection induced by the vaccine, the vaccine dose and the orthopoxvirus immune status of vaccine recipients. Recombinant viral vectors are under development as novel vaccine candidates that induce immunity to antigens of interest expressed from transgenes. -

Risk Groups: Viruses (C) 1988, American Biological Safety Association
Rev.: 1.0 Risk Groups: Viruses (c) 1988, American Biological Safety Association BL RG RG RG RG RG LCDC-96 Belgium-97 ID Name Viral group Comments BMBL-93 CDC NIH rDNA-97 EU-96 Australia-95 HP AP (Canada) Annex VIII Flaviviridae/ Flavivirus (Grp 2 Absettarov, TBE 4 4 4 implied 3 3 4 + B Arbovirus) Acute haemorrhagic taxonomy 2, Enterovirus 3 conjunctivitis virus Picornaviridae 2 + different 70 (AHC) Adenovirus 4 Adenoviridae 2 2 (incl animal) 2 2 + (human,all types) 5 Aino X-Arboviruses 6 Akabane X-Arboviruses 7 Alastrim Poxviridae Restricted 4 4, Foot-and- 8 Aphthovirus Picornaviridae 2 mouth disease + viruses 9 Araguari X-Arboviruses (feces of children 10 Astroviridae Astroviridae 2 2 + + and lambs) Avian leukosis virus 11 Viral vector/Animal retrovirus 1 3 (wild strain) + (ALV) 3, (Rous 12 Avian sarcoma virus Viral vector/Animal retrovirus 1 sarcoma virus, + RSV wild strain) 13 Baculovirus Viral vector/Animal virus 1 + Togaviridae/ Alphavirus (Grp 14 Barmah Forest 2 A Arbovirus) 15 Batama X-Arboviruses 16 Batken X-Arboviruses Togaviridae/ Alphavirus (Grp 17 Bebaru virus 2 2 2 2 + A Arbovirus) 18 Bhanja X-Arboviruses 19 Bimbo X-Arboviruses Blood-borne hepatitis 20 viruses not yet Unclassified viruses 2 implied 2 implied 3 (**)D 3 + identified 21 Bluetongue X-Arboviruses 22 Bobaya X-Arboviruses 23 Bobia X-Arboviruses Bovine 24 immunodeficiency Viral vector/Animal retrovirus 3 (wild strain) + virus (BIV) 3, Bovine Bovine leukemia 25 Viral vector/Animal retrovirus 1 lymphosarcoma + virus (BLV) virus wild strain Bovine papilloma Papovavirus/ -

Activity of the Anti-Orthopoxvirus Compound ST-246 Against Vaccinia, Cowpox and Camelpox Viruses in Cell Monolayers and Organotypic Raft Cultures
Andrei 21/11/07 15:46 Page 1205 Antiviral Therapy 12:1205–1216 Activity of the anti-orthopoxvirus compound ST-246 against vaccinia, cowpox and camelpox viruses in cell monolayers and organotypic raft cultures Sophie Duraffour1,2, Robert Snoeck1, Rita de Vos 3, Joost J van Den Oord3, Jean-Marc Crance 2, Daniel Garin2, Dennis E Hruby4, Robert Jordan4, Erik De Clercq1 and Graciela Andrei1* 1Rega Institute For Medical Research, KU Leuven, Leuven, Belgium 2CRSSA Emile Pardé, Virology Laboratory, La Tronche, France 3Pathology Department, UZ Leuven, Leuven, Belgium 4SIGA Technologies, Inc., Corvallis, Oregon, CA, USA *Corresponding author: Tel: +32 16 33 73 72; Fax: +32 16 33 73 40; E-mail: [email protected] Background: The potential use of variola virus as a biolog- Results: ST-246 inhibited preferentially the production of ical weapon has renewed efforts in the development of extracellular virus compared with intracellular virus antiviral agents against orthopoxviruses. ST-246 [4- production in HEL and PHK cells (for VV) and in PHK cells trifluoromethyl-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-di (for CMLV). In organotypic epithelial raft cultures, ST-246 oxo-4,6-ethenocycloprop [f]isoindol-2(1H)-yl)-benza- at 20 μg/ml inhibited extracellular VV and CMLV produc- mide] is an anti-orthopoxvirus compound active against tion by 6 logs, whereas intracellular virus yield was several orthopoxviruses including vaccinia virus (VV), reduced by 2 logs. In the case of CPV, both extracellular cowpox virus (CPV), camelpox virus (CMLV), ectromelia and intracellular virus production were completely inhib- virus (ECTV) and variola virus in cell culture. -

Introduction to Viroids and Prions
Harriet Wilson, Lecture Notes Bio. Sci. 4 - Microbiology Sierra College Introduction to Viroids and Prions Viroids – Viroids are plant pathogens made up of short, circular, single-stranded RNA molecules (usually around 246-375 bases in length) that are not surrounded by a protein coat. They have internal base-pairs that cause the formation of folded, three-dimensional, rod-like shapes. Viroids apparently do not code for any polypeptides (proteins), but do cause a variety of disease symptoms in plants. The mechanism for viroid replication is not thoroughly understood, but is apparently dependent on plant enzymes. Some evidence suggests they are related to introns, and that they may also infect animals. Disease processes may involve RNA-interference or activities similar to those involving mi-RNA. Prions – Prions are proteinaceous infectious particles, associated with a number of disease conditions such as Scrapie in sheep, Bovine Spongiform Encephalopathy (BSE) or Mad Cow Disease in cattle, Chronic Wasting Disease (CWD) in wild ungulates such as muledeer and elk, and diseases in humans including Creutzfeld-Jacob disease (CJD), Gerstmann-Straussler-Scheinker syndrome (GSS), Alpers syndrome (in infants), Fatal Familial Insomnia (FFI) and Kuru. These diseases are characterized by loss of motor control, dementia, paralysis, wasting and eventually death. Prions can be transmitted through ingestion, tissue transplantation, and through the use of comtaminated surgical instruments, but can also be transmitted from one generation to the next genetically. This is because prion proteins are encoded by genes normally existing within the brain cells of various animals. Disease is caused by the conversion of normal cell proteins (glycoproteins) into prion proteins. -

Herpesviral Latency—Common Themes
pathogens Review Herpesviral Latency—Common Themes Magdalena Weidner-Glunde * , Ewa Kruminis-Kaszkiel and Mamata Savanagouder Department of Reproductive Immunology and Pathology, Institute of Animal Reproduction and Food Research of Polish Academy of Sciences, Tuwima Str. 10, 10-748 Olsztyn, Poland; [email protected] (E.K.-K.); [email protected] (M.S.) * Correspondence: [email protected] Received: 22 January 2020; Accepted: 14 February 2020; Published: 15 February 2020 Abstract: Latency establishment is the hallmark feature of herpesviruses, a group of viruses, of which nine are known to infect humans. They have co-evolved alongside their hosts, and mastered manipulation of cellular pathways and tweaking various processes to their advantage. As a result, they are very well adapted to persistence. The members of the three subfamilies belonging to the family Herpesviridae differ with regard to cell tropism, target cells for the latent reservoir, and characteristics of the infection. The mechanisms governing the latent state also seem quite different. Our knowledge about latency is most complete for the gammaherpesviruses due to previously missing adequate latency models for the alpha and beta-herpesviruses. Nevertheless, with advances in cell biology and the availability of appropriate cell-culture and animal models, the common features of the latency in the different subfamilies began to emerge. Three criteria have been set forth to define latency and differentiate it from persistent or abortive infection: 1) persistence of the viral genome, 2) limited viral gene expression with no viral particle production, and 3) the ability to reactivate to a lytic cycle. This review discusses these criteria for each of the subfamilies and highlights the common strategies adopted by herpesviruses to establish latency.