Monkeypox Virus Emergence in Wild Chimpanzees Reveals Distinct Clinical Outcomes and Viral Diversity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Poxvirus and Rabies Branch (PRB) Fact Sheet
Poxvirus & Rabies Branch (PRB) The epidemiologists, laboratory scientists, and public health professionals in PRB are responsible for surveillance, control, and prevention of illness and death due to poxviruses and rabies. Our Work Laboratory Reference, Diagnostics, & Training PRB’s provides laboratory reference, diagnostic services, and trainings to state and local health departments, the Department of Defense (DOD), international organizations, and the Laboratory Response Network (over 150 labs across the U.S. prepared to respond to bioterrorism). PRB collaborates with the private sector to develop and evaluate novel diagnostic assays, therapeutics, and vaccines for rabies and pox-related viruses. The branch maintains high-containment laboratories (BSL3 &4) to safely conduct public health research. Research & Surveillance PRB conducts research studies on the microbiology, molecular biology, pathogenesis (disease process), ecology, and evolution of Our Pathogens poxviruses and rabies. The branch monitors the incidence rates of PRB’s Poxvirus Team provides subject matter these diseases in the U.S. and internationally. expertise on: Expert Consultation Orthopoxviruses, including PRB provides consultation regarding poxvirus and rabies- Smallpox associated diseases and their diagnoses, prevention, control, and Monkeypox treatments. Assistance is offered to healthcare providers, academic Cowpox institutions, state and local health departments, other government Vaccinia Virus agencies, and the general public. PRB collaborates with non- governmental organizations to develop risk communication Parapoxviruses, including tools for disease prevention. The branch provides training on Orf Virus simple surveillance techniques and trains healthcare workers on monkeypox in the Democratic Republic of the Congo (DRC). PRB Pseudocowpox collaborates with the national government of Haiti on a rabies surveillance and control program which includes health education, enhanced diagnostics, surveillance, and an extensive dog vaccination campaign. -

The Munich Outbreak of Cutaneous Cowpox Infection: Transmission by Infected Pet Rats
Acta Derm Venereol 2012; 92: 126–131 INVESTIGATIVE REPORT The Munich Outbreak of Cutaneous Cowpox Infection: Transmission by Infected Pet Rats Sandra VOGEL1, Miklós SÁRDY1, Katharina GLOS2, Hans Christian KOrting1, Thomas RUZICKA1 and Andreas WOLLENBERG1 1Department of Dermatology and Allergology, Ludwig Maximilian University, Munich, and 2Department of Dermatology, Haas and Link Animal Clinic, Germering, Germany Cowpox virus infection of humans is an uncommon, another type of orthopoxvirus, from infected pet prairie potentially fatal, skin disease. It is largely confined to dogs have recently been described in the USA, making Europe, but is not found in Eire, or in the USA, Austral the medical community aware of the risk of transmission asia, or the Middle or Far East. Patients having contact of pox viruses from pets (3). with infected cows, cats, or small rodents sporadically Seven of 8 exposed patients living in the Munich contract the disease from these animals. We report here area contracted cowpox virus infection from an unusual clinical aspects of 8 patients from the Munich area who source: rats infected with cowpox virus bought from had purchased infected pet rats from a local supplier. Pet local pet shops and reputedly from the same supplier rats are a novel potential source of local outbreaks. The caused a clinically distinctive pattern of infection, which morphologically distinctive skin lesions are mostly res was mostly restricted to the patients’ neck and trunk. tricted to the patients’ necks, reflecting the infected ani We report here dermatologically relevant aspects of mals’ contact pattern. Individual lesions vaguely resem our patients in order to alert the medical community to ble orf or Milker’s nodule, but show marked surrounding the possible risk of a zoonotic orthopoxvirus outbreak erythema, firm induration and local adenopathy. -

Where Do We Stand After Decades of Studying Human Cytomegalovirus?
microorganisms Review Where do we Stand after Decades of Studying Human Cytomegalovirus? 1, 2, 1 1 Francesca Gugliesi y, Alessandra Coscia y, Gloria Griffante , Ganna Galitska , Selina Pasquero 1, Camilla Albano 1 and Matteo Biolatti 1,* 1 Laboratory of Pathogenesis of Viral Infections, Department of Public Health and Pediatric Sciences, University of Turin, 10126 Turin, Italy; [email protected] (F.G.); gloria.griff[email protected] (G.G.); [email protected] (G.G.); [email protected] (S.P.); [email protected] (C.A.) 2 Complex Structure Neonatology Unit, Department of Public Health and Pediatric Sciences, University of Turin, 10126 Turin, Italy; [email protected] * Correspondence: [email protected] These authors contributed equally to this work. y Received: 19 March 2020; Accepted: 5 May 2020; Published: 8 May 2020 Abstract: Human cytomegalovirus (HCMV), a linear double-stranded DNA betaherpesvirus belonging to the family of Herpesviridae, is characterized by widespread seroprevalence, ranging between 56% and 94%, strictly dependent on the socioeconomic background of the country being considered. Typically, HCMV causes asymptomatic infection in the immunocompetent population, while in immunocompromised individuals or when transmitted vertically from the mother to the fetus it leads to systemic disease with severe complications and high mortality rate. Following primary infection, HCMV establishes a state of latency primarily in myeloid cells, from which it can be reactivated by various inflammatory stimuli. Several studies have shown that HCMV, despite being a DNA virus, is highly prone to genetic variability that strongly influences its replication and dissemination rates as well as cellular tropism. In this scenario, the few currently available drugs for the treatment of HCMV infections are characterized by high toxicity, poor oral bioavailability, and emerging resistance. -

Characterization of Host Micrornas That Respond to DNA Virus Infection in a Crustacean Tianzhi Huang, Dandan Xu and Xiaobo Zhang*
Huang et al. BMC Genomics 2012, 13:159 http://www.biomedcentral.com/1471-2164/13/159 RESEARCH ARTICLE Open Access Characterization of host microRNAs that respond to DNA virus infection in a crustacean Tianzhi Huang, Dandan Xu and Xiaobo Zhang* Abstract Background: MicroRNAs (miRNAs) are key posttranscriptional regulators of gene expression that are implicated in many processes of eukaryotic cells. It is known that the expression profiles of host miRNAs can be reshaped by viruses. However, a systematic investigation of marine invertebrate miRNAs that respond to virus infection has not yet been performed. Results: In this study, the shrimp Marsupenaeus japonicus was challenged by white spot syndrome virus (WSSV). Small RNA sequencing of WSSV-infected shrimp at different time post-infection (0, 6, 24 and 48 h) identified 63 host miRNAs, 48 of which were conserved in other animals, representing 43 distinct families. Of the identified host miRNAs, 31 were differentially expressed in response to virus infection, of which 25 were up-regulated and six down-regulated. The results were confirmed by northern blots. The TargetScan and miRanda algorithms showed that most target genes of the differentially expressed miRNAs were related to immune responses. Gene ontology analysis revealed that immune signaling pathways were mediated by these miRNAs. Evolutionary analysis showed that three of them, miR-1, miR-7 and miR-34, are highly conserved in shrimp, fruit fly and humans and function in the similar pathways. Conclusions: Our study provides the first large-scale characterization of marine invertebrate miRNAs that respond to virus infection. This will help to reveal the molecular events involved in virus-host interactions mediated by miRNAs and their evolution in animals. -

Specimen Type, Collection Methods, and Diagnostic Assays Available For
Specimen type, collection methods, and diagnostic assays available for the detection of poxviruses from human specimens by the Poxvirus and Rabies Branch, Centers for Disease Control and Prevention1. Specimen Orthopoxvirus Parapoxvirus Yatapoxvirus Molluscipoxvirus Specimen type collection method PCR6 Culture EM8 IHC9,10 Serology11 PCR12 EM8 IHC9,10 PCR13 EM8 PCR EM8 Lesion material Fresh or frozen Swab 5 Lesion material [dry or in media ] [vesicle / pustule Formalin fixed skin, scab / crust, etc.] Paraffin block Fixed slide(s) Container Lesion fluid Swab [vesicle / pustule [dry or in media5] fluid, etc.] Touch prep slide Blood EDTA2 EDTA tube 7 Spun or aliquoted Serum before shipment Spun or aliquoted Plasma before shipment CSF3,4 Sterile 1. The detection of poxviruses by electron microscopy (EM) and immunohistochemical staining (IHC) is performed by the Infectious Disease Pathology Branch of the CDC. 2. EDTA — Ethylenediaminetetraacetic acid. 3. CSF — Cerebrospinal fluid. 4. In order to accurately interpret test results generated from CSF specimens, paired serum must also be submitted. 5. If media is used to store and transport specimens a minimal amount should be used to ensure as little dilution of DNA as possible. 6. Orthopoxvirus generic real-time polymerase chain reaction (PCR) assays will amplify DNA from numerous species of virus within the Orthopoxvirus genus. Species-specific real-time PCR assays are available for selective detection of DNA from variola virus, vaccinia virus, monkeypox virus, and cowpox virus. 7. Blood is not ideal for the detection of orthopoxviruses by PCR as the period of viremia has often passed before sampling occurs. 8. EM can reveal the presence of a poxvirus in clinical specimens or from virus culture, but this technique cannot differentiate between virus species within the same genus. -
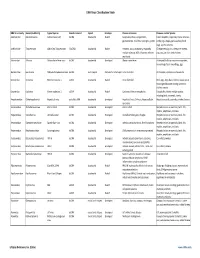
Virus Classification Tables V2.Vd.Xlsx
DNA Virus Classification Table DNA Virus Family Genera (Subfamily) Typical Species Genetic material Capsid Envelope Disease in Humans Diseases in other Species Adenoviridae Mastadenovirus Adenoviruses 1‐47 dsDNA Icosahedral Naked Respiratory illness; conjunctivitis, Canine hepatitis, respiratory illness in horses, gastroenteritis, tonsillitis, meningitis, cystitis cattle, pigs, sheep, goats, sea lions, birds dogs, squirrel enteritis Anelloviridae Torqueviruses Alpha‐Zeta Torqueviruses (‐)ssDNA Icosahedral Naked Hepatitis, lupus, pulmonary, myopathy, Chimpanzee, pig, cow, sheep, tree shrews, multiple sclerosis; 90% of humans infected pigs, cats, sea lions and chickens worldwide Asfarviridae Asfivirus African Swine fever virus dsDNA Icosahedral Enveloped African swine fever Arthropod (tick) transmission or ingestion; hemorrhagic fever in warthogs, pigs Baculoviridae Baculovirus Alpha‐Gamma Baculoviruses dsDNA Stick shaped Occluded or Enveloped none identified Arthropods, Lepidoptera, crustaceans Circoviridae Circovirus Porcine circovirus 1 ssDNA Icosahedral Naked none identified Birds, pigs, dogs; bats; rodents; causes post‐ weaning multisystem wasting syndrome, chicken anemia Circoviridae Cyclovirus Human cyclovirus 1 ssDNA Icosahedral Naked Cyclovirus Vietnam encephalitis Encephalitis; infects multiple species including birds, mammals, insects Hepadnaviridae Orthohepadnavirus Hepatitis B virus partially ssDNA Icosahedral Enveloped Hepatitis B virus; Cirrhosis, Hepatocellular Hepatitis in ducks, squirrels, primates, herons carcinoma Herpesviridae -

Imported Monkeypox, Singapore
DISPATCHES Imported Monkeypox, Singapore Sarah Ee Fang Yong, Oon Tek Ng, Zheng Jie Marc Ho, Tze Minn Mak, Kalisvar Marimuthu, Shawn Vasoo, Tsin Wen Yeo, Yi Kai Ng, Lin Cui, Zannatul Ferdous, Po Ying Chia, Bryan Jun Wei Aw, Charmaine Malenab Manauis, Constance Khia Ki Low, Guanhao Chan, Xinyi Peh, Poh Lian Lim, Li Ping Angela Chow, Monica Chan, Vernon Jian Ming Lee, Raymond Tzer Pin Lin, Mok Kwee Derrick Heng, Yee Sin Leo In May 2019, we investigated monkeypox in a traveler and public health management for this case, together from Nigeria to Singapore. The public health response with lessons learned and implications for control. included rapid identification of contacts, use of quaran- tine, and postexposure smallpox vaccination. No sec- The Case ondary cases were identified. Countries should develop On May 8, 2019, monkeypox was laboratory-con- surveillance systems to detect emerging infectious dis- firmed in a 38-year-old man from Nigeria who had eases globally. traveled to Singapore. The man resided in Delta State, Nigeria, but had attended a wedding in Ebonyi State onkeypox is a zoonosis endemic to West and during April 21–23, where he reported ingestion of MCentral Africa; human cases were first report- barbecued bushmeat that might have been contami- ed in 1970 (1). An outbreak ongoing in Nigeria since nated. He did not handle raw meat and had no expo- 2017 is the largest documented (2). Exported cases in sure to wild animals or their products. He held an ad- the United Kingdom and Israel were reported from ministrative job and reported no contact with rodents travelers infected in Nigeria in 2018 (3,4). -

Select Biological Agent and Toxin List
Select Biological Agent and Toxin List HHS Non-Overlap Select Agents and Toxins USDA High Consequence Livestock Pathogens - Crimean-Congo haemorrhagic fever virus and Toxins (Non-Overlap agents and toxins) - Coccidioides posadasii - Akabane virus - Ebola viruses - African swine fever virus - Cercopithecine herpesvirus 1 (Herpes B virus) - African horse sickness virus - Lassa fever virus - Avian influenza virus (highly pathogenic) - Marburg virus - Blue tongue virus (Exotic) - Monkeypox virus - Bovine spongiform encephalopathy agent - Rickettsia prowazekii - Camel pox virus - Rickettsia rickettsii - Classical swine fever virus South American haemorrhagic fever viruses - Cowdria ruminantium (Heartwater) - Junin - Foot and mouth disease virus - Machupo - Goat pox virus - Sabia - Lumpy skin disease virus - Flexal - Japanese encephalitis virus - Guanarito - Malignant catarrhal fever virus (Exotic) Tick-borne encephalitis complex (flavi) viruses - Menangle virus - Central European tick-borne encephalitis - Mycoplasma capricolum/M.F38/M. mycoides capri - Far Eastern tick-borne encephalitis - Mycoplasma mycoides mycoides - Russian spring and summer encephalitis - Newcastle disease virus (VVND) - Kyasanur forest disease - Peste Des Petits Ruminants virus - Omsk hemorrhagic fever - Rinderpest virus - Variola major virus (Smallpox virus) - Sheep pox virus - Variola minor virus (Alastrim) - Swine vesicular disease virus - Yersinia pestis - Vesicular stomatitis virus (Exotic) - Abrin - Conotoxins - Diacetoxyscirpenol Plant Pathogens - Ricin - Liberobacter -

Emergence of Monkeypox — West and Central Africa, 1970–2017
Morbidity and Mortality Weekly Report Emergence of Monkeypox — West and Central Africa, 1970–2017 Kara N. Durski, MPH1; Andrea M. McCollum, PhD2; Yoshinori Nakazawa, PhD2; Brett W. Petersen, MD2; Mary G. Reynolds, PhD2; Sylvie Briand, MD, PhD1; Mamoudou Harouna Djingarey, MD3; Victoria Olson, PhD2; Inger K. Damon, MD, PhD2, Asheena Khalakdina, PhD1 The recent apparent increase in human monkeypox cases Monkeypox is a zoonotic orthopoxvirus with a similar dis- across a wide geographic area, the potential for further spread, ease presentation to smallpox in humans, with the additional and the lack of reliable surveillance have raised the level of distinguishing symptom of lymphdenopathy. After an initial concern for this emerging zoonosis. In November 2017, the febrile prodrome, a centrifugally distributed maculopapular World Health Organization (WHO), in collaboration with rash develops, with lesions often present on the palms of CDC, hosted an informal consultation on monkeypox with the hands and soles of the feet. The infection can last up to researchers, global health partners, ministries of health, and 4 weeks, until crusts separate and a fresh layer of skin is formed. orthopoxvirus experts to review and discuss human monkeypox Sequelae include secondary bacterial infections, respiratory in African countries where cases have been recently detected and distress, bronchopneumonia, gastrointestinal involvement, also identify components of surveillance and response that need dehydration, encephalitis, and ocular infections, which can improvement. Endemic human monkeypox has been reported result in permanent corneal scarring. No specific treatment for from more countries in the past decade than during the previ- a monkeypox virus infection currently exists, and patients are ous 40 years. -

Effects of Pre-Existing Orthopoxvirus-Specific Immunity On
www.nature.com/scientificreports OPEN Efects of pre-existing orthopoxvirus-specifc immunity on the performance of Modifed Received: 4 January 2018 Accepted: 10 April 2018 Vaccinia virus Ankara-based Published: xx xx xxxx infuenza vaccines Arwen F. Altenburg1, Stella E. van Trierum1, Erwin de Bruin1, Dennis de Meulder1, Carolien E. van de Sandt1, Fiona R. M. van der Klis2, Ron A. M. Fouchier1, Marion P. G. Koopmans1, Guus F. Rimmelzwaan1,3 & Rory D. de Vries1 The replication-defcient orthopoxvirus modifed vaccinia virus Ankara (MVA) is a promising vaccine vector against various pathogens and has an excellent safety record. However, pre-existing vector- specifc immunity is frequently suggested to be a drawback of MVA-based vaccines. To address this issue, mice were vaccinated with MVA-based infuenza vaccines in the presence or absence of orthopoxvirus-specifc immunity. Importantly, protective efcacy of an MVA-based infuenza vaccine against a homologous challenge was not impaired in the presence of orthopoxvirus-specifc pre-existing immunity. Nonetheless, orthopoxvirus-specifc pre-existing immunity reduced the induction of antigen- specifc antibodies under specifc conditions and completely prevented induction of antigen-specifc T cell responses by rMVA-based vaccination. Notably, antibodies induced by vaccinia virus vaccination, both in mice and humans, were not capable of neutralizing MVA. Thus, when using rMVA-based vaccines it is important to consider the main correlate of protection induced by the vaccine, the vaccine dose and the orthopoxvirus immune status of vaccine recipients. Recombinant viral vectors are under development as novel vaccine candidates that induce immunity to antigens of interest expressed from transgenes. -

Serological Evidence of Multiple Zoonotic Viral Infections Among Wild Rodents in Barbados
pathogens Article Serological Evidence of Multiple Zoonotic Viral Infections among Wild Rodents in Barbados Kirk Osmond Douglas 1,*, Claire Cayol 2 , Kristian Michael Forbes 3, Thelma Alafia Samuels 4, Olli Vapalahti 5, Tarja Sironen 5 and Marquita Gittens-St. Hilaire 6,7 1 Centre for Biosecurity Studies, The University of the West Indies, Cave Hill, St. Michael BB11000, Barbados 2 Department of Wildlife, Fish, and Environmental Studies, Swedish University of Agricultural Sciences, Skogsmarksgränd 17, 901 83 Umeå, Sweden; [email protected] 3 Department of Biological Sciences, University of Arkansas, Fayetteville, AR 72701, USA; [email protected] 4 Epidemiology Research Unit, Caribbean Institute for Health Research (CAIHR), The University of the West Indies, Mona, Kingston 7, Jamaica; alafi[email protected] 5 Department of Virology, Faculty of Medicine, University of Helsinki, Medicum, Haartmaninkatu 3, 0290 Helsinki, Finland; olli.vapalahti@helsinki.fi (O.V.); tarja.sironen@helsinki.fi (T.S.) 6 Faculty of Medical Sciences, The University of the West Indies, Cave Hill, St. Michael BB11000, Barbados; [email protected] 7 Best–dos Santos Public Health Laboratory, Enmore #6, Lower Collymore Rock, St. Michael BB11155, Barbados * Correspondence: [email protected]; Tel.: +246-417-7468 Abstract: Background: Rodents are reservoirs for several zoonotic pathogens that can cause human infectious diseases, including orthohantaviruses, mammarenaviruses and orthopoxviruses. Evidence exists for these viruses circulating among rodents and causing human infections in the Americas, Citation: Douglas, K.O.; Cayol, C.; but much less evidence exists for their presence in wild rodents in the Caribbean. Methods: Here, Forbes, K.M.; Samuels, T.A.; we conducted serological and molecular investigations of wild rodents in Barbados to determine Vapalahti, O.; Sironen, T.; Gittens-St. -

Risk Groups: Viruses (C) 1988, American Biological Safety Association
Rev.: 1.0 Risk Groups: Viruses (c) 1988, American Biological Safety Association BL RG RG RG RG RG LCDC-96 Belgium-97 ID Name Viral group Comments BMBL-93 CDC NIH rDNA-97 EU-96 Australia-95 HP AP (Canada) Annex VIII Flaviviridae/ Flavivirus (Grp 2 Absettarov, TBE 4 4 4 implied 3 3 4 + B Arbovirus) Acute haemorrhagic taxonomy 2, Enterovirus 3 conjunctivitis virus Picornaviridae 2 + different 70 (AHC) Adenovirus 4 Adenoviridae 2 2 (incl animal) 2 2 + (human,all types) 5 Aino X-Arboviruses 6 Akabane X-Arboviruses 7 Alastrim Poxviridae Restricted 4 4, Foot-and- 8 Aphthovirus Picornaviridae 2 mouth disease + viruses 9 Araguari X-Arboviruses (feces of children 10 Astroviridae Astroviridae 2 2 + + and lambs) Avian leukosis virus 11 Viral vector/Animal retrovirus 1 3 (wild strain) + (ALV) 3, (Rous 12 Avian sarcoma virus Viral vector/Animal retrovirus 1 sarcoma virus, + RSV wild strain) 13 Baculovirus Viral vector/Animal virus 1 + Togaviridae/ Alphavirus (Grp 14 Barmah Forest 2 A Arbovirus) 15 Batama X-Arboviruses 16 Batken X-Arboviruses Togaviridae/ Alphavirus (Grp 17 Bebaru virus 2 2 2 2 + A Arbovirus) 18 Bhanja X-Arboviruses 19 Bimbo X-Arboviruses Blood-borne hepatitis 20 viruses not yet Unclassified viruses 2 implied 2 implied 3 (**)D 3 + identified 21 Bluetongue X-Arboviruses 22 Bobaya X-Arboviruses 23 Bobia X-Arboviruses Bovine 24 immunodeficiency Viral vector/Animal retrovirus 3 (wild strain) + virus (BIV) 3, Bovine Bovine leukemia 25 Viral vector/Animal retrovirus 1 lymphosarcoma + virus (BLV) virus wild strain Bovine papilloma Papovavirus/