Cross-Neutralizing and Protective Human Antibody Specificities To
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Accidental Vaccinia Virus Exposure: Information Sheet for Laboratory Workers
The University of Iowa Vaccinia Vaccination Information Form I understand that due to my occupational exposure to vaccinia virus, I may be at risk of acquiring infection from vaccinia. I have been given the opportunity to be vaccinated with the smallpox vaccine, at no charge to myself. After reading the Medication Guide and information packet from the Centers for Disease Control and Prevention (CDC) I understand there are risks involved in working with vaccinia virus, in addition to risks in receving the vaccination. My signature below indicates that I have: 1. Read the CDC’s Medication Guide that has been provided to me (follows this informational form). 2. Read the CDC’s "Important Information about Vaccinia (Smallpox) Vaccine For Laboratory Workers" that has been provided to me (follows this informational form). 3. Had an opportunity to ask questions about vaccinia with Biosafety Staff in the Environmental Health and Safety Office (EHS) at 335-8501. 4. Had the opportunity to ask questions about the vaccine with University Employee Health Clinic staff at 356-3631. I have decided to: Decline the vaccination at this time. I understand that by declining this vaccine, I continue to be at risk of acquiring an infection from vaccinia. If I continue to have occupational exposure to this virus and decide to be vaccinated in the future, I may request and receive the vaccination, at no charge to myself. Receive the vaccine, provided through the University Employee Health Clinic (UEHC). Signature: Please print name: Date: University ID number: Principal Investigator/Lab Director Name: If consenting, please also complete: Date of Birth: Job Title: List of duties and/or viruses used in lab that may cause exposure to vaccinia virus: Return page 1 of this form to: Associate Biosafety Officer, 100 EHS. -

The Munich Outbreak of Cutaneous Cowpox Infection: Transmission by Infected Pet Rats
Acta Derm Venereol 2012; 92: 126–131 INVESTIGATIVE REPORT The Munich Outbreak of Cutaneous Cowpox Infection: Transmission by Infected Pet Rats Sandra VOGEL1, Miklós SÁRDY1, Katharina GLOS2, Hans Christian KOrting1, Thomas RUZICKA1 and Andreas WOLLENBERG1 1Department of Dermatology and Allergology, Ludwig Maximilian University, Munich, and 2Department of Dermatology, Haas and Link Animal Clinic, Germering, Germany Cowpox virus infection of humans is an uncommon, another type of orthopoxvirus, from infected pet prairie potentially fatal, skin disease. It is largely confined to dogs have recently been described in the USA, making Europe, but is not found in Eire, or in the USA, Austral the medical community aware of the risk of transmission asia, or the Middle or Far East. Patients having contact of pox viruses from pets (3). with infected cows, cats, or small rodents sporadically Seven of 8 exposed patients living in the Munich contract the disease from these animals. We report here area contracted cowpox virus infection from an unusual clinical aspects of 8 patients from the Munich area who source: rats infected with cowpox virus bought from had purchased infected pet rats from a local supplier. Pet local pet shops and reputedly from the same supplier rats are a novel potential source of local outbreaks. The caused a clinically distinctive pattern of infection, which morphologically distinctive skin lesions are mostly res was mostly restricted to the patients’ neck and trunk. tricted to the patients’ necks, reflecting the infected ani We report here dermatologically relevant aspects of mals’ contact pattern. Individual lesions vaguely resem our patients in order to alert the medical community to ble orf or Milker’s nodule, but show marked surrounding the possible risk of a zoonotic orthopoxvirus outbreak erythema, firm induration and local adenopathy. -

Where Do We Stand After Decades of Studying Human Cytomegalovirus?
microorganisms Review Where do we Stand after Decades of Studying Human Cytomegalovirus? 1, 2, 1 1 Francesca Gugliesi y, Alessandra Coscia y, Gloria Griffante , Ganna Galitska , Selina Pasquero 1, Camilla Albano 1 and Matteo Biolatti 1,* 1 Laboratory of Pathogenesis of Viral Infections, Department of Public Health and Pediatric Sciences, University of Turin, 10126 Turin, Italy; [email protected] (F.G.); gloria.griff[email protected] (G.G.); [email protected] (G.G.); [email protected] (S.P.); [email protected] (C.A.) 2 Complex Structure Neonatology Unit, Department of Public Health and Pediatric Sciences, University of Turin, 10126 Turin, Italy; [email protected] * Correspondence: [email protected] These authors contributed equally to this work. y Received: 19 March 2020; Accepted: 5 May 2020; Published: 8 May 2020 Abstract: Human cytomegalovirus (HCMV), a linear double-stranded DNA betaherpesvirus belonging to the family of Herpesviridae, is characterized by widespread seroprevalence, ranging between 56% and 94%, strictly dependent on the socioeconomic background of the country being considered. Typically, HCMV causes asymptomatic infection in the immunocompetent population, while in immunocompromised individuals or when transmitted vertically from the mother to the fetus it leads to systemic disease with severe complications and high mortality rate. Following primary infection, HCMV establishes a state of latency primarily in myeloid cells, from which it can be reactivated by various inflammatory stimuli. Several studies have shown that HCMV, despite being a DNA virus, is highly prone to genetic variability that strongly influences its replication and dissemination rates as well as cellular tropism. In this scenario, the few currently available drugs for the treatment of HCMV infections are characterized by high toxicity, poor oral bioavailability, and emerging resistance. -

Characterization of Host Micrornas That Respond to DNA Virus Infection in a Crustacean Tianzhi Huang, Dandan Xu and Xiaobo Zhang*
Huang et al. BMC Genomics 2012, 13:159 http://www.biomedcentral.com/1471-2164/13/159 RESEARCH ARTICLE Open Access Characterization of host microRNAs that respond to DNA virus infection in a crustacean Tianzhi Huang, Dandan Xu and Xiaobo Zhang* Abstract Background: MicroRNAs (miRNAs) are key posttranscriptional regulators of gene expression that are implicated in many processes of eukaryotic cells. It is known that the expression profiles of host miRNAs can be reshaped by viruses. However, a systematic investigation of marine invertebrate miRNAs that respond to virus infection has not yet been performed. Results: In this study, the shrimp Marsupenaeus japonicus was challenged by white spot syndrome virus (WSSV). Small RNA sequencing of WSSV-infected shrimp at different time post-infection (0, 6, 24 and 48 h) identified 63 host miRNAs, 48 of which were conserved in other animals, representing 43 distinct families. Of the identified host miRNAs, 31 were differentially expressed in response to virus infection, of which 25 were up-regulated and six down-regulated. The results were confirmed by northern blots. The TargetScan and miRanda algorithms showed that most target genes of the differentially expressed miRNAs were related to immune responses. Gene ontology analysis revealed that immune signaling pathways were mediated by these miRNAs. Evolutionary analysis showed that three of them, miR-1, miR-7 and miR-34, are highly conserved in shrimp, fruit fly and humans and function in the similar pathways. Conclusions: Our study provides the first large-scale characterization of marine invertebrate miRNAs that respond to virus infection. This will help to reveal the molecular events involved in virus-host interactions mediated by miRNAs and their evolution in animals. -

ACAM2000 Clonal Vero Cell Culture Vaccinia Virus (New York City Board of Health Strain) — a Second-Generation Smallpox Vaccine for Biological Defense
International Journal of Infectious Diseases (2004) 8S2, S31—S44 http://intl.elsevierhealth.com/journals/ijid ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain) — a second-generation smallpox vaccine for biological defense Thomas P. Monatha,*, Joseph R. Caldwella, Wolfgang Mundtb, Joan Fuscob, Casey S. Johnsonc, Mark Bullerd, Jian Liua, Bridget Gardnera, Greg Downinga, Paul S. Bluma, Tracy Kempa, Richard Nicholsa, Richard Weltzina aAcambis Inc., 38, Sidney Street, Cambridge, MA 02139, USA bBaxter BioScience, USA and Austria cPRA International, Lenexa, KS dSt. Louis University Medical School, St. Louis MO Summary The threat of smallpox as a biological weapon has spurred efforts to create stockpiles of vaccine for emergency preparedness. In lieu of preparing vaccine in animal skin (the original method), we cloned vaccinia virus (New York City Board of Health strain, Dryvax1) by plaque purification and amplified the clone in cell culture. The overarching goal was to produce a modern vaccine that was equivalent to the currently licensed Dryvax1 in its preclinical and clinical properties, and could thus reliably protect humans against smallpox. A variety of clones were evaluated, and many were unacceptably virulent in animal models. One clonal virus (ACAM1000) was selected and produced at clinical grade in MRC-5 human diploid cells. ACAM1000 was comparable to Dryvax1 in immunogenicity and protective activity but was less neurovirulent for mice and nonhuman primates. To meet requirements for large quantities of vaccine after the events of September 11th 2001, the ACAM1000 master virus seed was used to prepare vaccine (designated ACAM2000) at large scale in Vero cells under serum-free conditions. -

Specimen Type, Collection Methods, and Diagnostic Assays Available For
Specimen type, collection methods, and diagnostic assays available for the detection of poxviruses from human specimens by the Poxvirus and Rabies Branch, Centers for Disease Control and Prevention1. Specimen Orthopoxvirus Parapoxvirus Yatapoxvirus Molluscipoxvirus Specimen type collection method PCR6 Culture EM8 IHC9,10 Serology11 PCR12 EM8 IHC9,10 PCR13 EM8 PCR EM8 Lesion material Fresh or frozen Swab 5 Lesion material [dry or in media ] [vesicle / pustule Formalin fixed skin, scab / crust, etc.] Paraffin block Fixed slide(s) Container Lesion fluid Swab [vesicle / pustule [dry or in media5] fluid, etc.] Touch prep slide Blood EDTA2 EDTA tube 7 Spun or aliquoted Serum before shipment Spun or aliquoted Plasma before shipment CSF3,4 Sterile 1. The detection of poxviruses by electron microscopy (EM) and immunohistochemical staining (IHC) is performed by the Infectious Disease Pathology Branch of the CDC. 2. EDTA — Ethylenediaminetetraacetic acid. 3. CSF — Cerebrospinal fluid. 4. In order to accurately interpret test results generated from CSF specimens, paired serum must also be submitted. 5. If media is used to store and transport specimens a minimal amount should be used to ensure as little dilution of DNA as possible. 6. Orthopoxvirus generic real-time polymerase chain reaction (PCR) assays will amplify DNA from numerous species of virus within the Orthopoxvirus genus. Species-specific real-time PCR assays are available for selective detection of DNA from variola virus, vaccinia virus, monkeypox virus, and cowpox virus. 7. Blood is not ideal for the detection of orthopoxviruses by PCR as the period of viremia has often passed before sampling occurs. 8. EM can reveal the presence of a poxvirus in clinical specimens or from virus culture, but this technique cannot differentiate between virus species within the same genus. -
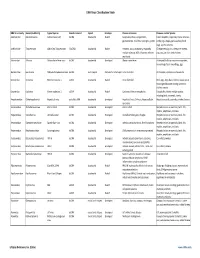
Virus Classification Tables V2.Vd.Xlsx
DNA Virus Classification Table DNA Virus Family Genera (Subfamily) Typical Species Genetic material Capsid Envelope Disease in Humans Diseases in other Species Adenoviridae Mastadenovirus Adenoviruses 1‐47 dsDNA Icosahedral Naked Respiratory illness; conjunctivitis, Canine hepatitis, respiratory illness in horses, gastroenteritis, tonsillitis, meningitis, cystitis cattle, pigs, sheep, goats, sea lions, birds dogs, squirrel enteritis Anelloviridae Torqueviruses Alpha‐Zeta Torqueviruses (‐)ssDNA Icosahedral Naked Hepatitis, lupus, pulmonary, myopathy, Chimpanzee, pig, cow, sheep, tree shrews, multiple sclerosis; 90% of humans infected pigs, cats, sea lions and chickens worldwide Asfarviridae Asfivirus African Swine fever virus dsDNA Icosahedral Enveloped African swine fever Arthropod (tick) transmission or ingestion; hemorrhagic fever in warthogs, pigs Baculoviridae Baculovirus Alpha‐Gamma Baculoviruses dsDNA Stick shaped Occluded or Enveloped none identified Arthropods, Lepidoptera, crustaceans Circoviridae Circovirus Porcine circovirus 1 ssDNA Icosahedral Naked none identified Birds, pigs, dogs; bats; rodents; causes post‐ weaning multisystem wasting syndrome, chicken anemia Circoviridae Cyclovirus Human cyclovirus 1 ssDNA Icosahedral Naked Cyclovirus Vietnam encephalitis Encephalitis; infects multiple species including birds, mammals, insects Hepadnaviridae Orthohepadnavirus Hepatitis B virus partially ssDNA Icosahedral Enveloped Hepatitis B virus; Cirrhosis, Hepatocellular Hepatitis in ducks, squirrels, primates, herons carcinoma Herpesviridae -

Study Protocol
Clinical Trial Protocol FY12-19, HP-12-19, POX-MVA-006 A randomized, open-label Phase III non-inferiority trial to compare indicators of efficacy for MVA-BN smallpox vaccine to ACAM2000 in 18-42 year old® healthy vaccinia naïve ®subjects Clinical Trial Protocol Edition 8 29 September 2016 NCT 01913353 Bavarian Nordic Page 1 of 1 Clinical Trial Protocol Doc. No. 92000007 FY12-19, HP-12-19, POX-MVA-006 Edition 8 FY12-19, HP-12-19, POX-MVA-006 A randomized, open-label Phase III non-inferiority trial to compare indicators of efficacy for MVA-BN smallpox vaccine to ACAM2000 in 18-42 year old® healthy vaccinia naïve ®subjects Clinical Trial Protocol Edition 8 29 September 2016 Bavarian Nordic Restricted Business Proprietary Page 1 of 208 Clinical Trial Protocol Doc. No. 92000007 FYI2-19, HP-12-19, POX-MVA-006 Edition 8 29-Sep-20 16 1 General Information 1.1 Principal Investigator Signature Page Herewith I agree that I have read and fully understand this protocol: A randomized, open-label Phase Ill non-inferiority trial to compare indicators of efficacy for MVA BN® smallpox vaccine to ACAM2000® in 18-42 year old healthy vaccinia-naiVe subjects This protocol describes all the information necessary to conduct the trial. I agree that I will conduct the trial according to the instructions given within this protocol. FUithermore, T agree that I will conduct this trial according to rnternational Conference of Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Good Clinical Practice (GCP), the 2013 version ofthe Declaration ofHelsinki, the Belmont Report, as well as applicable local legal and regulatory requirements in the respective countries. -

IMVANEX, Common Name – Modified Vaccinia Ankara Virus
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions. 1. NAME OF THE MEDICINAL PRODUCT IMVANEX suspension for injection Smallpox vaccine (Live Modified Vaccinia Virus Ankara) 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One dose (0.5 ml) contains: Modified Vaccinia Ankara – Bavarian Nordic Live virus1 no less than 5 x 107 Inf.U * *infectious units 1 Produced in chick embryo cells This vaccine contains trace residues of chicken protein, benzonase, gentamicin and ciprofloxacin (see section 4.3). For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Suspension for injection. Light yellow to pale white, milky suspension. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Active immunisation against smallpox in adults (see sections 4.4 and 5.1). The use of this vaccine should be in accordance with official recommendations. 4.2 Posology and method of administration Posology Primary vaccination (individuals previously not vaccinated against smallpox): A first dose of 0.5 ml should be administered on an elected date. A second dose of 0.5 ml should be administered no less than 28 days after the first dose. See sections 4.4 and 5.1. Booster vaccination (individuals previously vaccinated against smallpox): There are inadequate data to determine the appropriate timing of booster doses. If a booster dose is considered necessary then a single dose of 0.5 ml should be administered. See sections 4.4 and 5.1. -

IMVAMUNE® and ACAM2000® Provide Different Protection Against
Article IMVAMUNE® and ACAM2000® Provide Different Protection against Disease When Administered Postexposure in an Intranasal Monkeypox Challenge Prairie Dog Model M. Shannon Keckler 1,2 , Johanna S Salzer 1,3, Nishi Patel 1,4, Michael B Townsend 1, Yoshinori J Nakazawa 1, Jeffrey B Doty 1, Nadia F Gallardo-Romero 1, Panayampalli S Satheshkumar 1, Darin S Carroll 1,5, Kevin L Karem 1,6 and Inger K Damon 1,* 1 Poxvirus and Rabies Branch, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, Atlanta, GA 30333, USA; [email protected] (M.S.K.); [email protected] (J.S.S.); [email protected] (N.P.); [email protected] (M.B.T.); [email protected] (Y.J.N.); [email protected] (J.B.D.); [email protected] (N.F.G.-R.); [email protected] (P.S.S.); [email protected] (D.S.C.); [email protected] (K.L.K.) 2 Clinical and Environmental Microbiology Branch, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, Atlanta, GA 30333, USA 3 Bacterial Special Pathogens Branch, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, Atlanta, GA 30333, USA 4 Respiratory Diseases Branch, Division of Bacterial Diseases, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, Atlanta, GA 30333, USA 5 Office of the Director, National Center for Emerging and Zoonotic Diseases, Centers for Disease Control and Prevention, U.S. -

Monkeypox Virus Emergence in Wild Chimpanzees Reveals Distinct Clinical Outcomes and Viral Diversity
ARTICLES https://doi.org/10.1038/s41564-020-0706-0 Monkeypox virus emergence in wild chimpanzees reveals distinct clinical outcomes and viral diversity Livia V. Patrono1,6, Kamilla Pléh1,2,6, Liran Samuni2,3, Markus Ulrich1, Caroline Röthemeier1, Andreas Sachse1, Silvia Muschter4, Andreas Nitsche4, Emmanuel Couacy-Hymann5, Christophe Boesch2,3, Roman M. Wittig 2,3, Sébastien Calvignac-Spencer1 and Fabian H. Leendertz 1 ✉ Monkeypox is a viral zoonotic disease on the rise across endemic habitats. Despite the growing importance of monkeypox virus, our knowledge on its host spectrum and sylvatic maintenance is limited. Here, we describe the recent repeated emergence of monkeypox virus in a wild, human-habituated western chimpanzee (Pan troglodytes verus, hereafter chimpanzee) population from Taï National Park, Ivory Coast. Through daily monitoring, we show that further to causing its typical exanthematous syn- drome, monkeypox can present itself as a severe respiratory disease without a diffuse rash. By analysing 949 non-invasively collected samples, we identify the circulation of at least two distinct monkeypox virus lineages and document the shedding of infectious particles in faeces and flies, suggesting that they could mediate indirect transmission. We also show that the carnivo- rous component of the Taï chimpanzees’ diet, mainly consisting of the sympatric monkeys they regularly hunt, did not change nor shift towards rodent consumption (the presumed reservoir) before the outbreaks, suggesting that the sudden emergence of monkeypox virus in this population is probably due to changes in the ecology of the virus itself. Using long-term mortality surveillance data from Taï National Park, we provide evidence of little to no prior viral activity over at least two decades. -

Guidelines for Vaccination of Adult Solid Organ Transplant Candidates and Recipients
Stanford Healthcare Vaccination Subcommittee Issue Date: 7/2018 Guidelines for Vaccination of Adult Solid Organ Transplant Candidates and Recipients A. General considerations regarding vaccination 1. Adult solid organ transplant (SOT) candidates and recipients should receive all vaccines indicated based on their ages, medical conditions, and other factors that apply to non- SOT candidates or recipients (see http://www.cdc.gov/vaccines/schedules/hcp/adult.html, Appendix A, and Appendix B) except for the below-listed exceptions or additions. • All SOT candidates and recipients should be vaccinated against pneumococcus with PCV13 and PPSV23. • All SOT candidates and recipients should receive a HepB vaccine series with post-vaccination titers unless they have a documented anti-HBs titer of ≥ 10 mIU/mL after a properly-timed HepB series or unless they have known hepatitis B virus infection. • All SOT candidates and recipients should receive a HepA vaccine series unless previously administered or unless they have a positive hepatitis A virus immunoglobulin G assay. • Live-attenuated vaccines1 should not be administered to SOT recipients, SOT candidates on immunosuppression, or SOT candidates who may undergo SOT within 4 weeks. The timing of inactivated, subunit, or toxoid vaccines is discussed below. Vaccine Notes Influenza Can be given pre- and/or post-SOT (see Appendix C) Tdap or Td Can be given pre- and/or post-SOT Live-attenuated vaccine to be given pre-SOT only; generally those born MMR before 1957 (among others) are considered immune