Base Your Answers to Questions 1 and 2 on the Diagram Below, Which Shows the Results of Three Different Physical Tests, A, B, and C, That Were Performed on a Mineral
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Zincite (Zn, Mn2+)O
Zincite (Zn, Mn2+)O c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Hexagonal. Point Group: 6mm. Crystals rare, typically pyramidal, hemimorphic, with large {0001}, to 2.5 cm, rarely curved; in broad cleavages, foliated, granular, compact, massive. Twinning: On {0001}, with composition plane {0001}. Physical Properties: Cleavage: {1010}, perfect; parting on {0001}, commonly distinct. Fracture: Conchoidal. Tenacity: Brittle. Hardness = 4 VHN = 205–221 (100 g load). D(meas.) = 5.66(2) D(calc.) = 5.6730 Rare pale yellow fluorescence under LW UV. Optical Properties: Translucent, transparent in thin fragments. Color: Yellow-orange to deep red, rarely yellow, green, colorless; deep red to yellow in transmitted light; light rose-brown in reflected light, with strong red to yellow internal reflections. Streak: Yellow-orange. Luster: Subadamantine to resinous. Optical Class: Uniaxial (+). ω = 2.013 = 2.029 R1–R2: (400) 13.0–13.6, (420) 12.8–13.2, (440) 12.6–12.8, (460) 12.3–12.6, (480) 12.1–12.4, (500) 12.0–12.2, (520) 11.8–12.1, (540) 11.8–12.0, (560) 11.7–11.9, (580) 11.6–11.8, (600) 11.4–11.7, (620) 11.3–11.6, (640) 11.2–11.5, (660) 11.1–11.4, (680) 11.0–11.2, (700) 11.0–11.2 Cell Data: Space Group: P 63mc (synthetic). a = 3.24992(5) c = 5.20658(8) Z = 2 X-ray Powder Pattern: Synthetic. 2.476 (100), 2.816 (71), 2.602 (56), 1.626 (40), 1.477 (35), 1.911 (29), 1.379 (28) Chemistry: (1) (2) SiO2 0.08 FeO 0.01 0.23 MnO 0.27 0.29 ZnO 99.63 98.88 Total 99.99 [99.40] (1) Sterling Hill, New Jersey, USA. -

Download the Scanned
American Mineralogist, Volume 70, pages 379-387, 1985 Ma_nganesehumites and leucophoenicitesfrom Franklin and Sterling- Hill' NewJersev: 'i"? andimplications ;ifi':il1,;;lfiil11"r's' Perr J. Dullx Department of Mineral Sciences Smithsonian lnstitution, Washington, D. C. 20560 Abstract The manganesehumites, (alleghanyite, manganhumite, and sonolite),together with some Mn-bearing samplesof the Mg-humites,and the related phasesleucophoenicite and jerry- gibbsite,from the orebodiesat Franklin and SterlingHill, New Jersey,are describedtogether with analytical data. Solid solution betweenhumite and manganhumiteis at least partially continuous. Expected Mn/Mg solid solutions between alleghanyiteand chondrodite, and betweensonolite and clinohumite, are discontinuous; they are interrupted by apparently orderedphases. In all cases,the possibleorderings involve Zn as well as Mn and Mg. There are no Mn end-membersof the manganesehumites at this locality. Manganeseis apparently restricted in leucophoenicite(5.42-6.63 Mn per 7 octahedral cations) and in jerrygibbsite (7.79-8.02Mn per 9 octahedralcations). Calcium is common to both leucophoeniciteand jerrygibbsite,but among the Mn-humites,only sonoliteaccepts appreciable Ca (0.65Ca per 9 octahedralcations). There is a "threshold" level ofzinc in all studiedsamples; this "threshold" levelis a constantfor leucophoenicite1-9.3 Znper 3 Si)and alleghanyite(-O.2Zn per 2 Si). No samplesof leucophoeniciteor jerrygibbsite were found to be Zn-ftee,suggesting either that Zn is required for their stability, or that these two phasesmight not be stable as end-members.Fluorine is present in all the Mn-humites and is proportional to the Mg- content,but is absentin leucophoeniciteand jerrygibbsite. Introduction humite speciesoccur there; the Mg-humites occur in the The magnesiumhumite species(norbergite, chondrodite, host Franklin Marble for the most part, and the Mn- humite, and clinohumite) have been well-studiedand re- humites in the orebodiesthemselves. -

Leucophoenicite Mn (Sio4)3(OH)2
2+ Leucophoenicite Mn7 (SiO4)3(OH)2 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Monoclinic. Point Group: 2=m: Crystals rare, typically slender, prismatic, elongated and striated [010], to 8 mm; in isolated grains or granular massive. Twinning: On k 001 , common, contact or interpenetrant twins, lamellar. f g Physical Properties: Cleavage: 001 , imperfect. Tenacity: Brittle. Hardness = 5.5{6 f g D(meas.) = 3.848 D(calc.) = [4.01] Optical Properties: Transparent to translucent. Color: Brown to light purple-red, raspberry-red, deep pink to light pink; rose-red to colorless in thin section. Luster: Vitreous. Optical Class: Biaxial ({). Pleochroism: Faint; rose-red 001 ; colorless 001 . Orientation: k f g ? f g X 001 cleavage. Dispersion: r > v; slight. ® = 1.751(3) ¯ = 1.771(3) ° = 1.782(3) ? f g 2V(meas.) = 74(5)± Cell Data: Space Group: P 21=a: a = 10.842(19) b = 4.826(6) c = 11.324(9) ¯ = 103:93(9)± Z = [2] X-ray Powder Pattern: Franklin, New Jersey, USA. 1.8063 (10), 2.877 (9), 2.684 (8), 4.36 (5), 3.612 (5), 2.365 (5), 2.620 (4) Chemistry: (1) (2) (3) (1) (2) (3) SiO2 26.36 26.7 26.7 CaO 5.67 2.4 2.8 FeO trace 0.3 0.3 Na2O 0.39 MnO 60.63 62.8 64.7 K2O 0.24 ZnO 3.87 0.0 0.0 H2O 2.64 [2.3] [2.8] MgO 0.21 5.5 2.7 Total 100.01 [100.0] [100.0] (1) Franklin, New Jersey, USA; composite of two analyses, corresponding to (Mn5:89Ca0:70Zn0:32 Na0:04Mg0:03K0:01)§=6:99(Si1:01O4)3(OH)2: (2) Kombat mine, Namibia; by electron microprobe, H2O by di®erence; corresponding to (Mn5:98Mg0:92Ca0:29Fe0:02)§=7:21(SiO4)3(OH)1:72: (3) Valsesia-Valtournanche area, Italy; by electron microprobe, H2O by di®erence; corresponding to (Mn6:16Mg0:45Ca0:34Fe0:03)§=6:98(SiO4)3(OH)2:10: Mineral Group: Leucophoenicite group. -

TEPHROITE from FRANKLIN, NEW JERSEY* Connbrrus S. Hunrsur
THE AMERICAN MINERALOGIST, VOL 46, MAY_JUNE, 1961 TEPHROITE FROM FRANKLIN, NEW JERSEY* ConNBrrus S. Hunrsur, Jn., Departmentof Mineralogy, Harvard, Uniaersity. Assrnlcr A study of tephroite specimens from Franklin and Sterling Hill, New Jersey showed in all of them the presence of thin sheets of willemite believed to be a product of exsolution. .fhese sheets are oriented parallel to the {100} and [010] planes of tephroite with the o and r axes of tephroite and willemite parallel. It is believed that Iittle zinc remains in the tephroite structure and that much of it reported in chemical analyses has been con- tributed by intergrown u'illemite. This conclusion is supported by experiments syn- thesizing tephroite. The indices of refraction and d spacing of {130} vary as would be expected with changes in amounts of MgO, FeO and CaO. INrnooucrroN 'fephroite, Mn2SiO4,a member of the olivine group, was describedas a new mineral from SterlingHill by Breithaupt in 1823.A chemicalanal- ysis of the original material was published by Brush (1864) together with severaladditional chemical analysesof tephroite made by others. These analysesreport ZnO in varying amounts which Brush attributed to invariably associatedzincite. Palache (1937) did not agree with Brush and stated " . that the molecularratios in someanalyses more nearly satisfy the orthosilicateformula when zinc is regardedas essen- tially a part of the mineral rather than as a constituent of mechanical inclusions." The present study was undertaken for the purpose of in- vestigatingthe variations in the propertiesof tephroite with changesin chemical composition, particularly the effect of zinc. Relationships were not expectedto be simplefor analysesshow, in addition to ZnO, variable amounts of MgO, FeO, and CaO. -
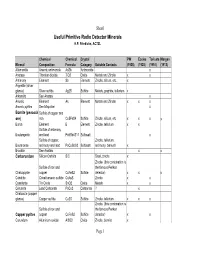
Useful Primitive Radio Detector Minerals H.P
Sheet1 Useful Primitive Radio Detector Minerals H.P. Friedichs, AC7ZL Chemical Chemical Crystal PW Eccles Toricata Morgan Mineral Composition Formula Category Suitable Contacts (1925) (1928) (1910) (1913) Allemontite Arsenic antimonide AsSb Antimonide x Anatase Titanium dioxide TiO2 Oxide Metals and Zincite x x Antimony Element Sb Element Zincite, silicon, etc. x Argentite (silver glance) Silver sulfide Ag2S Sulfide Metals, graphite, tellurium x Arkansite See Anatase x Arsenic Element As Element Metals and Zincite x x x Arsenic pyrites See Mispickel x Bornite (peacock Sulfide of copper and ore) iron Cu5FeS4 Sulfide Zincite, silicon, etc. x x x x Boron Element B Element Zincite, tellurium x x Sulfide of antimony Boulangerite and lead Pb5Sb4S11 Sulfosalt x Sulfide of copper, Zincite, tellurium, Bourmonite antimony and lead PbCuSbS3 Sulfosalt antimony, bismuth x Brookite See Anatase x x Carborundum Silicon Carbide SiC Steel, zincite x Zincite (this combination is Sulfide of iron and the famous Perikon Chalcopyrite copper CuFeS2 Sulfide detector) x x x Cobaltite Cobalt arsenic sulfide CoAsS Zincite x x Cassiterite Tin Oxide SnO2 Oxide Metals x x Cerussite Lead Carbonate PbCo3 Carbonate ?x Chalcocite (copper glance) Copper sulfide Cu2S Sulfide Zincite, tellurium x x x Zincite (this combination is Sulfide of iron and the famous Perikon Copper pyrites copper CuFeS2 Sulfide detector) x x Corundum Aluminum oxidde Al2O3 Oxide Zincite, bornite x Page 1 Sheet1 Covellite Copper sulfide CuS Sulfide Zincite, etc x x Cuprite (Cuprous oxide) Copper oxide -

Jerrygibbsite Mn (Sio4)
2+ Jerrygibbsite Mn9 (SiO4)4(OH)2 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Orthorhombic. Point Group: mm2 (probable), or 2=m 2=m 2=m: As interlocking anhedral crystals, to 2 mm. Physical Properties: Cleavage: Imperfect on 001 . Hardness = 5.5 D(meas.) = 4.00(2) D(calc.) = 4.045 f g » Optical Properties: Transparent and translucent lamellae alternating 001 . Color: Violet-pink, with a brownish tinge; light pink in thin section. Strekakf: Light pink. Luster: Vitreous. Optical Class: Biaxial ({). Orientation: X = b; Y = c; Z = a. Dispersion: r > v; moderate. ® = 1.772(4) ¯ = 1.783(4) ° = 1.789(4) 2V(meas.) = 72± Cell Data: Space Group: P bn21 (probable), or P bnm: a = 4.875(2) b = 10.709(6) c = 28.18(2) Z = 4 X-ray Powder Pattern: Franklin, New Jersey, USA. 2.557 (100), 1.806 (100), 2.869 (78), 2.752 (49), 2.702 (46), 2.362 (39), 2.661 (34) Chemistry: (1) SiO2 27.1 FeO 0.3 MnO 64.1 ZnO 3.9 MgO 1.4 CaO 0.4 F 0.0 H2O 2.13 Total 99.3 (1) Franklin, New Jersey, USA; by electron microprobe, H2O by the Pen¯eld method; corresponds to (Mn7:86Zn0:59Mg0:24Ca0:16Fe0:14)§=8:99(SiO4)4(OH)2: Polymorphism & Series: Dimorphous with sonolite. Mineral Group: Leucophoenicite group. Occurrence: In a metamorphosed stratiform Zn-Mn deposit. Association: Franklinite, willemite, zincite, sonolite, leucophoenicite, tephroite. Distribution: From Franklin, Sussex Co., New Jersey, USA. Name: In honor of Professor Gerald V. Gibbs, Virginia Polytechnic Institute and State University, Blacksburg, Virginia, USA. -

Identification of 1850S Brown Zinc Paint Made with Franklinite and Zincite at the U.S
Identification of 1850s Brown Zinc Paint Made with Franklinite and Zincite at the U.S. Capitol Author(s): Frank S. Welsh Source: APT Bulletin, Vol. 39, No. 1 (2008), pp. 17-30 Published by: Association for Preservation Technology International (APT) Stable URL: http://www.jstor.org/stable/25433934 Accessed: 25/12/2009 10:57 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/action/showPublisher?publisherCode=aptech. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Association for Preservation Technology International (APT) is collaborating with JSTOR to digitize, preserve and extend access to APT Bulletin. http://www.jstor.org Identification of 1850s Brown Zinc Paint Made with Franklinite and Zincite at the U.S. -

Gahnite-Franklinite Intergrowths at the Sterling Hill Zinc Deposit, Sussex County, New Jersey: an Analytical and Experimental Study
Lehigh University Lehigh Preserve Theses and Dissertations 1-1-1978 Gahnite-franklinite intergrowths at the Sterling Hill zinc deposit, Sussex County, New Jersey: An analytical and experimental study. Antone V. Carvalho Follow this and additional works at: http://preserve.lehigh.edu/etd Part of the Geology Commons Recommended Citation Carvalho, Antone V., "Gahnite-franklinite intergrowths at the Sterling Hill zinc deposit, Sussex County, New Jersey: An analytical and experimental study." (1978). Theses and Dissertations. Paper 2136. This Thesis is brought to you for free and open access by Lehigh Preserve. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of Lehigh Preserve. For more information, please contact [email protected]. GAHNITE-FRANKLINITE INTERGROWTHS AT THE STERLING HILL ZINC DEPOSIT, SUSSEX COUNTY, NEW JERSEY: AN ANALYTICAL AND EXPERIMENTAL STUDY by Antone V. Carvalho III A Thesis Presented to the Graduate Committee of Lehigh University in Candidacy for the Degree of Master of Science in Geological Sciences Lehigh University 1978 ProQuest Number: EP76409 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest EP76409 Published by ProQuest LLC (2015). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code Microform Edition © ProQuest LLC. ProQuest LLC. -

Bulletin 65, the Minerals of Franklin and Sterling Hill, New Jersey, 1962
THEMINERALSOF FRANKLINAND STERLINGHILL NEWJERSEY BULLETIN 65 NEW JERSEYGEOLOGICALSURVEY DEPARTMENTOF CONSERVATIONAND ECONOMICDEVELOPMENT NEW JERSEY GEOLOGICAL SURVEY BULLETIN 65 THE MINERALS OF FRANKLIN AND STERLING HILL, NEW JERSEY bY ALBERT S. WILKERSON Professor of Geology Rutgers, The State University of New Jersey STATE OF NEw JERSEY Department of Conservation and Economic Development H. MAT ADAMS, Commissioner Division of Resource Development KE_rr_ H. CR_V_LINCDirector, Bureau of Geology and Topography KEMBLEWIDX_, State Geologist TRENTON, NEW JERSEY --1962-- NEW JERSEY GEOLOGICAL SURVEY NEW JERSEY GEOLOGICAL SURVEY CONTENTS PAGE Introduction ......................................... 5 History of Area ................................... 7 General Geology ................................... 9 Origin of the Ore Deposits .......................... 10 The Rowe Collection ................................ 11 List of 42 Mineral Species and Varieties First Found at Franklin or Sterling Hill .......................... 13 Other Mineral Species and Varieties at Franklin or Sterling Hill ............................................ 14 Tabular Summary of Mineral Discoveries ................. 17 The Luminescent Minerals ............................ 22 Corrections to Franklln-Sterling Hill Mineral List of Dis- credited Species, Incorrect Names, Usages, Spelling and Identification .................................... 23 Description of Minerals: Bementite ......................................... 25 Cahnite .......................................... -

Behavior of Zn-Bearing Phases in Base Metal Slag from France and Poland: a Mineralogical Approach for Environmental Purposes
Journal of Geochemical Exploration 136 (2014) 1–13 Contents lists available at ScienceDirect Journal of Geochemical Exploration journal homepage: www.elsevier.com/locate/jgeoexp Behavior of Zn-bearing phases in base metal slag from France and Poland: A mineralogical approach for environmental purposes Maxime Vanaecker a, Alexandra Courtin-Nomade a,⁎,HubertBrila, Jacky Laureyns b,Jean-FrançoisLenaina a Université de Limoges, GRESE, E.A. 4330, IFR 145 GEIST, F.S.T., 123 Avenue A. Thomas, 87060 Limoges Cedex, France b Université de Lille 1, USTL, LASIR, C5, BP 69, 59652 Villeneuve d'Ascq Cedex, France article info abstract Article history: Slag samples from three pyrometallurgical sites (two in France, one in Poland) were studied for their Zn-phase con- Received 18 February 2013 tent, evolution and potential release of metals over time. Mineral assemblages were observed and analyzed using Accepted 3 September 2013 various complementary tools and approaches: chemical extractions, optical microscopy, cathodoluminescence, Available online 12 September 2013 X-ray diffraction, Scanning Electron Microscopy, ElectronProbeMicro-Analysis,andmicro-Raman spectrometry. The primary assemblages are composed of analogs to willemite, hardystonite, zincite, wurtzite, petedunnite and Keywords: franklinite. Some of these phases are sensitive to alteration (e.g., deuteric processes during cooling and by Slag fi Weathering weathering) and, as a result, goslarite, smithsonite and hemimorphite have been identi ed as secondary products. Zn-phase stability In comparing these results to the geochemical conditions at each site in relation to mineralogical investigations, Melilites different steps of Zn-rich mineral destabilization could be identified. This procedure allows assessing potential Raman spectroscopy environmental impacts due to a release of metals that may contain slag. -

Gageite (Mn ; Mg; Zn)42Si16o54(OH)40 C 2001 Mineral Data Publishing, Version 1.2 ° Crystal Data: Monoclinic, Pseudotetragonal, Or Triclinic
2+ Gageite (Mn ; Mg; Zn)42Si16O54(OH)40 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Monoclinic, pseudotetragonal, or triclinic. Point Group: 2=m or 1: Minute laths or saddlelike crystals grouped radially, in bundles, or as matted ¯bers. Physical Properties: Cleavage: 110 , pronounced. Hardness = 3 D(meas.) = 3.46{3.584 D(calc.) = 3.599 f g Optical Properties: Transparent to translucent. Color: Colorless, pale pink, pale brown. Luster: Highly vitreous. Optical Class: Biaxial ({). Orientation: Z ¯ber length. Dispersion: r < v; extreme. ® = 1.723(3) ¯ = 1.734(3) ° = 1.736(3) k2V(meas.) = n.d. Cell Data: Space Group: P 2=n: a = 19.42 b = 19.42 c = 9.84 ¯ = 89:5± Z = [1], or Space Group: P 1: a = 14.17 b = 14.07 c = 9.84 ® = 76:5± ¯ = 76:6± ° = 86:9± Z = [1] X-ray Powder Pattern: Franklin, New Jersey, USA. 6.87 (100), 2.758 (80), 2.707 (80), 3.44 (60), 3.25 (60), 2.556 (60), 1.6742 (60) Chemistry: (1) (2) (3) SiO2 24.71 23.58 23.90 Al2O3 0.15 FeO 0.03 0.20 MnO 50.19 53.74 51.06 ZnO 8.76 3.96 4.30 MgO 11.91 9.95 11.34 CaO 0.19 H2O [4.43] 8.24 [9.01] Total [100.00] 99.65 [100.00] (1) Franklin, New Jersey, USA; H2O by di®erence. (2) Do. (3) Do.; by electron microprobe, average of seven analyses, H2O by di®erence; corresponds to (Mn28:95Mg11:32Zn2:13 Ca0:14Fe0:11)§=42:65Si16O54:53(OH)40:23: Polymorphism & Series: 2M, 1A polytypes. -

6 Lead and Zinc
Energy and Environmental Profile of the U.S. Mining Industry 6 Lead and Zinc Lead and zinc ores are usually found together with gold and silver. A lead-zinc ore may also contain lead sulfide, zinc sulfide, iron sulfide, iron carbonate, and quartz. When zinc and lead sulfides are present in profitable amounts they are regarded as ore minerals. The remaining rock and minerals are called gangue. Forms of Lead and Zinc Ore The two principal minerals containing lead and zinc are galena and sphalerite. These two minerals are frequently found together along with other sulfide minerals, but one or the other may be predominant. Galena may contain small amounts of impurities including the precious metal silver, usually in the form of a sulfide. When silver is present in sufficient quantities, galena is regarded as a silver ore and called argentiferous galena. Sphalerite is zinc sulfide, but may contain iron. Black sphalerite may contain as much as 18 percent iron. Lead Ore The lead produced from lead ore is a soft, flexible and ductile metal. It is bluish-white, very dense, and has a low melting point. Lead is found in veins and masses in limestone and dolomite. It is also found with deposits of other metals, such as zinc, silver, copper, and gold. Lead is essentially a co-product of zinc mining or a byproduct of copper and/or gold and silver mining. Complex ores are also the source of byproduct metals such as bismuth, antimony, silver, copper, and gold. The most common lead-ore mineral is galena, or lead sulfide (PbS).