Formation of Large Synthetic Zincite (Zno) Crystals During Production of Zinc White
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 CRYSTAL CHEMISTRY of SELECTED Sb, As and P MINERALS
Crystal Chemistry of Selected Sb, As, and P Minerals Item Type text; Electronic Dissertation Authors Origlieri, Marcus Jason Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 07/10/2021 10:49:41 Link to Item http://hdl.handle.net/10150/194240 1 CRYSTAL CHEMISTRY OF SELECTED Sb, As AND P MINERALS by Marcus Jason Origlieri ___________________________________________ A Dissertation Submitted to the Faculty of the DEPARTMENT OF GEOSCIENCES In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY In the Graduate College THE UNIVERSITY OF ARIZONA 2005 2 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Marcus Jason Origlieri entitled Crystal Chemistry of Selected Sb, As, and P Minerals and recommend that it be accepted as fulfilling the dissertation requirement for the Degree of Doctor of Philosophy _______________________________________________________________________ Date: November 15, 2005 Robert T. Downs _______________________________________________________________________ Date: November 15, 2005 M. Bonner Denton _______________________________________________________________________ Date: November 15, 2005 Mihai N. Ducea _______________________________________________________________________ Date: November 15, 2005 Charles T. Prewitt Final approval and acceptance of this dissertation is contingent upon the candidate’s submission of the final copies of the dissertation to the Graduate College. I hereby certify that I have read this dissertation prepared under my direction and recommend that it be accepted as fulfilling the dissertation requirement. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Mineralogy and Geology of the \Vkgnerite Occurrence Co Santa Fe Mountain, Front Range, Cobrado
Mineralogy and Geology of the \Vkgnerite Occurrence co Santa Fe Mountain, Front Range, Cobrado GEOLOGICAL SURVEY PROFESSIONAL PAPER 955 Mineralogy and Geology of the Wignerite Occurance on Santa Fe Mountain, Front Range, Colorado By DOUGLAS M. SHERIDAN, SHERMAN P. MARSH, MARY E. MROSE, and RICHARD B. TAYLOR GEOLOGICAL SURVEY PROFESSIONAL PAPER 955 A detailed mineralogic study of wagnerite, a rare phosphate mineral occurring in the report area in Precambrian gneiss; this is the first recorded occurrence of wagnerite in the United States UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1976 UNITED STATES DEPARTMENT OF THE INTERIOR THOMAS S. KLEPPE, Secretary GEOLOGICAL SURVEY V. E. McKelvey, Director Library of Congress Cataloging in Publication Data Main entry under title: Mineralogy and geology of the wagnerite occurrence on Santa Fe Mountain, Front Range, Colorado. (Geological Survey Professional Paper 955) Includes bibliographical references. 1. Wagnerite Colorado Santa Fe Mountain. 2. Geology Colorado Santa Fe Mountain. I. Sheridan, Douglas M., 1921- II. Series: United States Geological Survey Professional Paper 955. QE391.W3M56 549'.72 76-10335 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, B.C. 20402 Stock Number 024-001-02844-1 CONTENTS Page Metric-English equivalents .............................. Descriptive mineralogy Continued Page Abstract............................................................ 1 Wagnerite............................................... 5 Introduction.................................................... -

Zincite (Zn, Mn2+)O
Zincite (Zn, Mn2+)O c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Hexagonal. Point Group: 6mm. Crystals rare, typically pyramidal, hemimorphic, with large {0001}, to 2.5 cm, rarely curved; in broad cleavages, foliated, granular, compact, massive. Twinning: On {0001}, with composition plane {0001}. Physical Properties: Cleavage: {1010}, perfect; parting on {0001}, commonly distinct. Fracture: Conchoidal. Tenacity: Brittle. Hardness = 4 VHN = 205–221 (100 g load). D(meas.) = 5.66(2) D(calc.) = 5.6730 Rare pale yellow fluorescence under LW UV. Optical Properties: Translucent, transparent in thin fragments. Color: Yellow-orange to deep red, rarely yellow, green, colorless; deep red to yellow in transmitted light; light rose-brown in reflected light, with strong red to yellow internal reflections. Streak: Yellow-orange. Luster: Subadamantine to resinous. Optical Class: Uniaxial (+). ω = 2.013 = 2.029 R1–R2: (400) 13.0–13.6, (420) 12.8–13.2, (440) 12.6–12.8, (460) 12.3–12.6, (480) 12.1–12.4, (500) 12.0–12.2, (520) 11.8–12.1, (540) 11.8–12.0, (560) 11.7–11.9, (580) 11.6–11.8, (600) 11.4–11.7, (620) 11.3–11.6, (640) 11.2–11.5, (660) 11.1–11.4, (680) 11.0–11.2, (700) 11.0–11.2 Cell Data: Space Group: P 63mc (synthetic). a = 3.24992(5) c = 5.20658(8) Z = 2 X-ray Powder Pattern: Synthetic. 2.476 (100), 2.816 (71), 2.602 (56), 1.626 (40), 1.477 (35), 1.911 (29), 1.379 (28) Chemistry: (1) (2) SiO2 0.08 FeO 0.01 0.23 MnO 0.27 0.29 ZnO 99.63 98.88 Total 99.99 [99.40] (1) Sterling Hill, New Jersey, USA. -

Download the Scanned
164 THE AMERICAN MINERAI,OGIST TABLE 3 Tlaln ro ssow Mnrnoo or Cer,cur,rfioN oF Axer,ns (SeeWi,nkeltabellen, pp. 18, 19 & 19a). Mlneral Etggttrslte Elements Do:1.272 I€t, Symb, lg Po: 010449 pq qo: .7940 lcp lgq lc so:989982 o I 0 0 01044e oaoosz I p+I 969897 0 e80346 e8es82 I 2 017609 030103 028058nrF,osR I 020085O200R5 qqo :te lpo p lg@:lgtanp lgsin 9 lg cos 9 I sn @ owQ qqo :19 tan p lrom 8 lrom 8 trcm 5 lrom I 3-6:4-7 020467 992858 972381 5802', 017591 56018', 017601 990364 979605 989233 38 42 000741 45 30 000749 007973 988573 980595 50 14 039485 68 04 039490 LISTS OF THE ORTIIORIIOMBIC MINERALS INCLUDED IN GOLDSCIIMIDT'S WINKELTABELLEN. Eocen T. WEONNY. WASh. ington, D. C.-As the prism zone is on the whole most cha.racteristicof orthor- hombic crystals, it has seemeddesirable to arrange the minerals of this system in the order of increasins values of axis a, Onrsonsolmrc MTNERALS ac Page a c Page Uranophanite......0.311.01 355 Tooaz. .....0.53 0.95 346 Polycrasite (Poly- Puiherite..........0.53l.l7 274 kras)...........0.35 0.31 27r Phosohosiderite....0.53 0.88 266 Euxenite ...0.36 0.30 r37 .Iordinite ...0.54 l.oz 191 Molybdite....:....0.39 0,47 243 Yttrobantalite......0.54 1.13 371 Columbite ..0.40 0.36 101 Rammelsbergite....0.54 - 291 Oanneroedite (An- Samarskite.........0.550.52 309 ner<idit).........0.400.36 45 Struvite ....0.55 0.62 332 Flinkite . -

New Mineral Names*
American Mineralogist, Volume 73, pages 1492-1499. 1988 NEW MINERAL NAMES* JOHN L. JAMBOR CANMET, 555 Booth Street, Ottawa, Ontario KIA OGI, Canada ERNST A. J. BURKE lnstituut voor Aardwetenschappen, Vrije Universitiete, De Boelelaan 1085, 1081 HV, Amsterdam, Netherlands T. SCOTT ERCIT, JOEL D. GRICE National Museum of Natural Sciences, Ottawa, Ontario KIA OM8, Canada Acuminite* prismatic to acicular crystals that are up to 10 mm long and 0.5 H. Pauly, O.Y. Petersen (1987) Acuminite, a new Sr-fluoride mm in diameter, elongate and striated [001], rhombic to hex- from Ivigtut, South Greenland. Neues Jahrb. Mineral. Mon., agonal in cross section, showing {l00} and {l10}. Perfect {100} 502-514. cleavage, conchoidal fracture, vitreous luster, H = 4, Dm'.. = 2.40(5) glcm3 (pycnometer), Dcale= 2.380 glcm3 for the ideal Wet-chemical analysis gave Li 0.0026, Ca 0.0185, Sr 37.04, formula, and Z = 4. Optically biaxial positive, a = 1.5328(4), (3 Al 11.86, F 33.52, OH (calc. from anion deficit) 6.82, H20 (calc. = 1.5340(4), 1.5378(4), 2 Vmoa,= 57(2)°, 2 Vcale= 59°; weak assuming 1 H20 in the formula) 7.80, sum 97.06 wt%, corre- 'Y = dispersion, r < v; Z = b, Y A c = -10°. X-ray structural study sponding to Sro98AIl.o2F.o7(OH)o.93H20. The mineral occurs as indicated monoclinic symmetry, space group C21c, a = 18.830(2), aggregates of crystals shaped like spear points and about I mm b= I 1.517(2), c= 5.190(I)A,{3 = 100.86(1)°. A Guinierpowder long. -

Download the Scanned
American Mineralogist, Volume 70, pages 379-387, 1985 Ma_nganesehumites and leucophoenicitesfrom Franklin and Sterling- Hill' NewJersev: 'i"? andimplications ;ifi':il1,;;lfiil11"r's' Perr J. Dullx Department of Mineral Sciences Smithsonian lnstitution, Washington, D. C. 20560 Abstract The manganesehumites, (alleghanyite, manganhumite, and sonolite),together with some Mn-bearing samplesof the Mg-humites,and the related phasesleucophoenicite and jerry- gibbsite,from the orebodiesat Franklin and SterlingHill, New Jersey,are describedtogether with analytical data. Solid solution betweenhumite and manganhumiteis at least partially continuous. Expected Mn/Mg solid solutions between alleghanyiteand chondrodite, and betweensonolite and clinohumite, are discontinuous; they are interrupted by apparently orderedphases. In all cases,the possibleorderings involve Zn as well as Mn and Mg. There are no Mn end-membersof the manganesehumites at this locality. Manganeseis apparently restricted in leucophoenicite(5.42-6.63 Mn per 7 octahedral cations) and in jerrygibbsite (7.79-8.02Mn per 9 octahedralcations). Calcium is common to both leucophoeniciteand jerrygibbsite,but among the Mn-humites,only sonoliteaccepts appreciable Ca (0.65Ca per 9 octahedralcations). There is a "threshold" level ofzinc in all studiedsamples; this "threshold" levelis a constantfor leucophoenicite1-9.3 Znper 3 Si)and alleghanyite(-O.2Zn per 2 Si). No samplesof leucophoeniciteor jerrygibbsite were found to be Zn-ftee,suggesting either that Zn is required for their stability, or that these two phasesmight not be stable as end-members.Fluorine is present in all the Mn-humites and is proportional to the Mg- content,but is absentin leucophoeniciteand jerrygibbsite. Introduction humite speciesoccur there; the Mg-humites occur in the The magnesiumhumite species(norbergite, chondrodite, host Franklin Marble for the most part, and the Mn- humite, and clinohumite) have been well-studiedand re- humites in the orebodiesthemselves. -

Leucophoenicite Mn (Sio4)3(OH)2
2+ Leucophoenicite Mn7 (SiO4)3(OH)2 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Monoclinic. Point Group: 2=m: Crystals rare, typically slender, prismatic, elongated and striated [010], to 8 mm; in isolated grains or granular massive. Twinning: On k 001 , common, contact or interpenetrant twins, lamellar. f g Physical Properties: Cleavage: 001 , imperfect. Tenacity: Brittle. Hardness = 5.5{6 f g D(meas.) = 3.848 D(calc.) = [4.01] Optical Properties: Transparent to translucent. Color: Brown to light purple-red, raspberry-red, deep pink to light pink; rose-red to colorless in thin section. Luster: Vitreous. Optical Class: Biaxial ({). Pleochroism: Faint; rose-red 001 ; colorless 001 . Orientation: k f g ? f g X 001 cleavage. Dispersion: r > v; slight. ® = 1.751(3) ¯ = 1.771(3) ° = 1.782(3) ? f g 2V(meas.) = 74(5)± Cell Data: Space Group: P 21=a: a = 10.842(19) b = 4.826(6) c = 11.324(9) ¯ = 103:93(9)± Z = [2] X-ray Powder Pattern: Franklin, New Jersey, USA. 1.8063 (10), 2.877 (9), 2.684 (8), 4.36 (5), 3.612 (5), 2.365 (5), 2.620 (4) Chemistry: (1) (2) (3) (1) (2) (3) SiO2 26.36 26.7 26.7 CaO 5.67 2.4 2.8 FeO trace 0.3 0.3 Na2O 0.39 MnO 60.63 62.8 64.7 K2O 0.24 ZnO 3.87 0.0 0.0 H2O 2.64 [2.3] [2.8] MgO 0.21 5.5 2.7 Total 100.01 [100.0] [100.0] (1) Franklin, New Jersey, USA; composite of two analyses, corresponding to (Mn5:89Ca0:70Zn0:32 Na0:04Mg0:03K0:01)§=6:99(Si1:01O4)3(OH)2: (2) Kombat mine, Namibia; by electron microprobe, H2O by di®erence; corresponding to (Mn5:98Mg0:92Ca0:29Fe0:02)§=7:21(SiO4)3(OH)1:72: (3) Valsesia-Valtournanche area, Italy; by electron microprobe, H2O by di®erence; corresponding to (Mn6:16Mg0:45Ca0:34Fe0:03)§=6:98(SiO4)3(OH)2:10: Mineral Group: Leucophoenicite group. -

TEPHROITE from FRANKLIN, NEW JERSEY* Connbrrus S. Hunrsur
THE AMERICAN MINERALOGIST, VOL 46, MAY_JUNE, 1961 TEPHROITE FROM FRANKLIN, NEW JERSEY* ConNBrrus S. Hunrsur, Jn., Departmentof Mineralogy, Harvard, Uniaersity. Assrnlcr A study of tephroite specimens from Franklin and Sterling Hill, New Jersey showed in all of them the presence of thin sheets of willemite believed to be a product of exsolution. .fhese sheets are oriented parallel to the {100} and [010] planes of tephroite with the o and r axes of tephroite and willemite parallel. It is believed that Iittle zinc remains in the tephroite structure and that much of it reported in chemical analyses has been con- tributed by intergrown u'illemite. This conclusion is supported by experiments syn- thesizing tephroite. The indices of refraction and d spacing of {130} vary as would be expected with changes in amounts of MgO, FeO and CaO. INrnooucrroN 'fephroite, Mn2SiO4,a member of the olivine group, was describedas a new mineral from SterlingHill by Breithaupt in 1823.A chemicalanal- ysis of the original material was published by Brush (1864) together with severaladditional chemical analysesof tephroite made by others. These analysesreport ZnO in varying amounts which Brush attributed to invariably associatedzincite. Palache (1937) did not agree with Brush and stated " . that the molecularratios in someanalyses more nearly satisfy the orthosilicateformula when zinc is regardedas essen- tially a part of the mineral rather than as a constituent of mechanical inclusions." The present study was undertaken for the purpose of in- vestigatingthe variations in the propertiesof tephroite with changesin chemical composition, particularly the effect of zinc. Relationships were not expectedto be simplefor analysesshow, in addition to ZnO, variable amounts of MgO, FeO, and CaO. -

POUDRETTEITE, Knarb3si12o3e, a NEW MEMBER of the OSUMILITE GROUP from MONT SAINT-HILAIRE, OUEBEC, and ITS CRVSTAL STRUCTURE Assr
Canadian Mineralogist Vol. 25, pp.763-166(1987) POUDRETTEITE,KNarB3Si12O3e, A NEW MEMBEROF THE OSUMILITEGROUP FROM MONT SAINT-HILAIRE,OUEBEC, AND ITS CRVSTALSTRUCTURE JOEL D. GRICE, T. SCOTT ERCIT AND JERRY VAN VELTHUIZEN Mineral SciencesDivision, National Museumof Natural Sciences,Ottawa, Ontario KIA 0M8 PETE J. DUNN Departmentof Mineral Sciences,Smithsonian Institution, Washington,D.C, 20560,U,S.A. Assrnact positionC d coordinanceXII, le sodium,la position,4d unecoordinance VI, le bore,la position72 ir coordinance Poudretteiteis a newmineral species from the Poudrette IV, le silicium,la position71 i coordinanceIV, etla posi- quarry, Mont Saint-Hilaire,Quebec. It occursin a marble tion B estvacante. xenolith includedin nephelinesyenite, associated with pec- tolite, apophyllite, quartz and minor aegirine.It forms clear, Mots-clds: poudrettdite, nouvelle espbce min6rale, groupe colorlessto very pale pink, equidimensional,subhedral del'osumilite, mont Saint-Hilaire, borosilicate, affi ne. prismsup to 5 mm. It is brittle, H about 5, with a splin- mentde Ia structure. tery fracture;Dmetr. 2.51(l) g/cml, D"6".2.53 g/cm3. Uniaxialpositive, co 1.516(l), e 1.532(l).It is-hexagonal, spacegroup P6/ mcc, a I 0.239(l), c 13.485(3)A and,Z : 2. INTRoDUcTIoN The strongestten X-ray-diffractionlines in the powderpat- tern [d in A(r\(hkDl are: 6.74(30)(002),5.13 (100)(110), The optical and physical propertiesof members 4.07(30)(r 12), 3.70(30)(202), 3.3 6e(30)(004), 3.253 ( I 00) of the osumilite group are similar to those of com- (2r r), 2.9s6(40)(3 00), 2.8 I 5(60)(I I 4), 2.686(50)(213,204) mon mineralssuch as quartz and cordierite;for this and 2.013(30)(321).An analysisby electron microprobe reason, they are probably generally overlooked. -
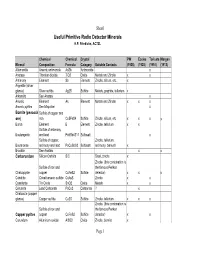
Useful Primitive Radio Detector Minerals H.P
Sheet1 Useful Primitive Radio Detector Minerals H.P. Friedichs, AC7ZL Chemical Chemical Crystal PW Eccles Toricata Morgan Mineral Composition Formula Category Suitable Contacts (1925) (1928) (1910) (1913) Allemontite Arsenic antimonide AsSb Antimonide x Anatase Titanium dioxide TiO2 Oxide Metals and Zincite x x Antimony Element Sb Element Zincite, silicon, etc. x Argentite (silver glance) Silver sulfide Ag2S Sulfide Metals, graphite, tellurium x Arkansite See Anatase x Arsenic Element As Element Metals and Zincite x x x Arsenic pyrites See Mispickel x Bornite (peacock Sulfide of copper and ore) iron Cu5FeS4 Sulfide Zincite, silicon, etc. x x x x Boron Element B Element Zincite, tellurium x x Sulfide of antimony Boulangerite and lead Pb5Sb4S11 Sulfosalt x Sulfide of copper, Zincite, tellurium, Bourmonite antimony and lead PbCuSbS3 Sulfosalt antimony, bismuth x Brookite See Anatase x x Carborundum Silicon Carbide SiC Steel, zincite x Zincite (this combination is Sulfide of iron and the famous Perikon Chalcopyrite copper CuFeS2 Sulfide detector) x x x Cobaltite Cobalt arsenic sulfide CoAsS Zincite x x Cassiterite Tin Oxide SnO2 Oxide Metals x x Cerussite Lead Carbonate PbCo3 Carbonate ?x Chalcocite (copper glance) Copper sulfide Cu2S Sulfide Zincite, tellurium x x x Zincite (this combination is Sulfide of iron and the famous Perikon Copper pyrites copper CuFeS2 Sulfide detector) x x Corundum Aluminum oxidde Al2O3 Oxide Zincite, bornite x Page 1 Sheet1 Covellite Copper sulfide CuS Sulfide Zincite, etc x x Cuprite (Cuprous oxide) Copper oxide -

Gem Quality Johachidolite: Occurrence, Chemical Composition and Crystal Structure
No.5 November 2007 GEM QUALITY JOHACHIDOLITE: OCCURRENCE, CHEMICAL COMPOSITION AND CRYSTAL STRUCTURE MESSAGE Editor Message from the Editor’s Desk Dr. A. Peretti, FGG, FGA, EurGeol GRS Gemresearch Swisslab AG, P.O.Box 4028, The continuous research on new gem occurrences 6002 Lucerne, Switzerland and new gemstones is a very important aspect of [email protected] the activities of a modern gemological laboratory. With the description of a revised crystal structure Previous Journals of painite in our previous publication and the description of the new gem mineral pezzottaite, GRS has invested much in this tradition. It has featured its discoveries in two issues of Contribution to Gemology No. 2 and No. 3 and published the detailed results in American Mineralogist and Mineralogical Record (see literature). Research institutions all over the world, some of them considered the most prestigious in this field of research such as Caltech University, have made links to the online version of our journal at www.gemresearch.ch, particularly suitable for students to appreciate our extensive use of illustrations. Every day several thousand visitors search our website from more than 15 countries, confirming the interest in rare collector gems in the gem community and the public. It took GRS and its collaborating scientific partners in Switzerland almost two years of intensive research to make another important discovery of corresponding caliber. Again, it is a discovery in the field of new gem occurrences and new important data on mineral structures and mineral chemistry. This time the news is not coming from new mines in Madagascar but from the famous valley of Mogok in Burma, renowned for its fabulous rubies and sapphires.