Download the Scanned
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Washington State Minerals Checklist
Division of Geology and Earth Resources MS 47007; Olympia, WA 98504-7007 Washington State 360-902-1450; 360-902-1785 fax E-mail: [email protected] Website: http://www.dnr.wa.gov/geology Minerals Checklist Note: Mineral names in parentheses are the preferred species names. Compiled by Raymond Lasmanis o Acanthite o Arsenopalladinite o Bustamite o Clinohumite o Enstatite o Harmotome o Actinolite o Arsenopyrite o Bytownite o Clinoptilolite o Epidesmine (Stilbite) o Hastingsite o Adularia o Arsenosulvanite (Plagioclase) o Clinozoisite o Epidote o Hausmannite (Orthoclase) o Arsenpolybasite o Cairngorm (Quartz) o Cobaltite o Epistilbite o Hedenbergite o Aegirine o Astrophyllite o Calamine o Cochromite o Epsomite o Hedleyite o Aenigmatite o Atacamite (Hemimorphite) o Coffinite o Erionite o Hematite o Aeschynite o Atokite o Calaverite o Columbite o Erythrite o Hemimorphite o Agardite-Y o Augite o Calciohilairite (Ferrocolumbite) o Euchroite o Hercynite o Agate (Quartz) o Aurostibite o Calcite, see also o Conichalcite o Euxenite o Hessite o Aguilarite o Austinite Manganocalcite o Connellite o Euxenite-Y o Heulandite o Aktashite o Onyx o Copiapite o o Autunite o Fairchildite Hexahydrite o Alabandite o Caledonite o Copper o o Awaruite o Famatinite Hibschite o Albite o Cancrinite o Copper-zinc o o Axinite group o Fayalite Hillebrandite o Algodonite o Carnelian (Quartz) o Coquandite o o Azurite o Feldspar group Hisingerite o Allanite o Cassiterite o Cordierite o o Barite o Ferberite Hongshiite o Allanite-Ce o Catapleiite o Corrensite o o Bastnäsite -

TRAVERTINE-MARL DEPOSITS of the VALLEY and RIDGE PROVINCE of VIRGINIA - a PRELIMINARY REPORT David A
- Vol. 31 February 1985 No. 1 TRAVERTINE-MARL DEPOSITS OF THE VALLEY AND RIDGE PROVINCE OF VIRGINIA - A PRELIMINARY REPORT David A. Hubbard, Jr.1, William F. Gianninil and Michelle M. Lorah2 The travertine and marl deposits of Virginia's Valley and Ridge province are the result of precipitation of calcium carbonate from fresh water streams and springs. Travertine is white to light yellowish brown and has a massive or concretionary structure. Buildups of this material tend to form cascades or waterfalls along streams (Figure 1). Marl refers to white to dark yellowish brown, loose, earthy deposits of calcium carbonate (Figure 2). Deposits of these carbonate materials are related and have formed during the Quaternary period. This preliminary report is a compilation of some litei-ature and observations of these materials. A depositional model is proposed. These deposits have long been visited by man. Projectile points, pottery fragments, and firepits record the visitation of American Indians to Frederick and Augusta county sites. Thomas Jefferson (1825) wrote an account of the Falling Spring Falls from a visit prior to 1781. Aesthetic and economic considerations eontinue to attract interest in these deposits. 'Virginia Division of Mineral Resources, Charlot- Figure 1. Travertine waterfall and cascade series tesville, VA on Falling Springs Creek, Alleghany County, 2Department of Environmental Sciences, Univer- Virginia. Note man standing in center of left sity of Virginia, Charlottesville, VA margin. 2 VIRGINIA DIVISION OF MINERAL RESOURCES Vol. 31 Figure 2. An extensive marl deposit located in Figure 3. Rimstone dam form resulting from Frederick County, Virginia. Stream, in fore- precipitation of calcium carbonate in Mill Creek, ground, has incised and drained the deposit. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

The Picking Table Volume 27, No. 1 – Spring 1986
TABLE JOURNAL of the FRANKLIN-OGDENSBURG MINERALOGICAL SOCIETY, INC. SPRING. 1986 VOLUME 27, NO.l The contents of The Picking Table are licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. F.QM.S. Notes prise a spectacular fluorescent display. For PRESIDENT'S MESSAGE years the Gerstmann Mineral Museum has displayed the collection for the delight and With the melting of the snow, the rocks of education of amateur and professional mineralo- the Buckwheat Dump emerge from their white gists alike. The Franklin Mineral Museum mantle, and the seismic tremors rumble through is most grateful to Arthur and Harriet Mitteldorf the souls of the collector community. Whatever for this most generous donation and to Ewald Spring may mean to the average mortal, to Gerstmann for its accumulation and for his FOMS members it brings a special appeal to sponsorship of the Franklin Mineral Museum dig in the dirt, not to plant, but to explore as the recipient. Transfer of the collection again the crystalline mysteries of Nature. will be effected as soon as suitable space is available to house it. Let us not lose sight of the fact that we are a community, however widespread, dedicated JLB to a great common interest and purpose: the expansion and preservation of knowledge about the world's most remarkable mineral location. ABOUT THE COVER SKETCH Like all great enterprises, this demands the efforts and participation of many. To the Located Sphalerite Occurrences—Franklin Mine extent that we share our knowledge, our time, and our interest with each other and the world, It is suggested that you refer to this hand Franklin lives. -

MINERALS and MINERAL VARIETIES from METAMORPHOSED Mn DEPOSITS of BISTRITA MOUNTAINS, ROMANIA
Acta Mineralogica-Petrographica, Abstract Series 1, Szeged, 2003 MINERALS AND MINERAL VARIETIES FROM METAMORPHOSED Mn DEPOSITS OF BISTRITA MOUNTAINS, ROMANIA HÎRTOPANU, P.1 & SCOTT, P.2 1 Geological Institute of Romania, Caransebeş 1, RO-78344 Bucharest, Romania. E-mail: [email protected] 2 Camborne School of Mines, Redruth, Cornwall, United Kingdom. The Bistrita Mountains belong to the Crystalline Meso- mation of some amphiboles and some pyroxenes into other zoic Zone of the East Carpathians, which consists of super- phases, there are drastical transformations of pyroxenes into posed Variscan and Alpine Nappes, overthrusted eastwards pyroxenoids (johannsenite into rhodonite), pyroxenoids into over the Flysch Zone. The manganese ore is contained by pyroxenoids (pyroxmangite into rhodonite), pyroxmangite Tulghes Group (Tg2 level) of the Variscan Putna Nappe, into manganogrunerite, garnets into garnets (spessartine- situated over the Pietrosu Bistritei Nappe and supporting the calderite into spessartine, spessartine into anisotropic spes- thrusting of the Rebra Nappe. All these Variscan nappes sartine-andradite-grossular), calderite into pyroxmangite- constitute the Alpine Sub-Bucovinian Nappe localised be- magnetite, etc. are the best evidences of continuous variation tween Alpine Infrabucovinian Nappe in the East and the of formation conditions. Alpine Bucovinian Nappe in the West. The Mn ore have a predominant carbonate rather than The mineralogy of Mn metamorphosed deposits from silicate mineralogical composition, which means a great CO2 Bistrita Mts. includes 328 minerals and mineral varieties. fluid control in the carbonation and dehydration processes They may count among the mineralogically the most com- along the many stages of the whole history of the ore and the plex deposits of the world. -

Zincite (Zn, Mn2+)O
Zincite (Zn, Mn2+)O c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Hexagonal. Point Group: 6mm. Crystals rare, typically pyramidal, hemimorphic, with large {0001}, to 2.5 cm, rarely curved; in broad cleavages, foliated, granular, compact, massive. Twinning: On {0001}, with composition plane {0001}. Physical Properties: Cleavage: {1010}, perfect; parting on {0001}, commonly distinct. Fracture: Conchoidal. Tenacity: Brittle. Hardness = 4 VHN = 205–221 (100 g load). D(meas.) = 5.66(2) D(calc.) = 5.6730 Rare pale yellow fluorescence under LW UV. Optical Properties: Translucent, transparent in thin fragments. Color: Yellow-orange to deep red, rarely yellow, green, colorless; deep red to yellow in transmitted light; light rose-brown in reflected light, with strong red to yellow internal reflections. Streak: Yellow-orange. Luster: Subadamantine to resinous. Optical Class: Uniaxial (+). ω = 2.013 = 2.029 R1–R2: (400) 13.0–13.6, (420) 12.8–13.2, (440) 12.6–12.8, (460) 12.3–12.6, (480) 12.1–12.4, (500) 12.0–12.2, (520) 11.8–12.1, (540) 11.8–12.0, (560) 11.7–11.9, (580) 11.6–11.8, (600) 11.4–11.7, (620) 11.3–11.6, (640) 11.2–11.5, (660) 11.1–11.4, (680) 11.0–11.2, (700) 11.0–11.2 Cell Data: Space Group: P 63mc (synthetic). a = 3.24992(5) c = 5.20658(8) Z = 2 X-ray Powder Pattern: Synthetic. 2.476 (100), 2.816 (71), 2.602 (56), 1.626 (40), 1.477 (35), 1.911 (29), 1.379 (28) Chemistry: (1) (2) SiO2 0.08 FeO 0.01 0.23 MnO 0.27 0.29 ZnO 99.63 98.88 Total 99.99 [99.40] (1) Sterling Hill, New Jersey, USA. -

Kumtyubeite Ca5(Sio4)2F2—A New Calcium Mineral of the Humite Group from Northern Caucasus, Kabardino-Balkaria, Russia
American Mineralogist, Volume 94, pages 1361–1370, 2009 Kumtyubeite Ca5(SiO4)2F2—A new calcium mineral of the humite group from Northern Caucasus, Kabardino-Balkaria, Russia Ir I n a О. Ga l u s k I n a ,1,* BI l j a n a la z I c ,2 Th o m a s ar m B r u s T e r,2 ev G e n y v. Ga l u s k I n ,1 vI k T o r m. Ga z e e v,3 al e k s a n d e r e. za d o v,4 nI k o l a I n. Pe r T s e v,3 lIdIa je ż a k ,5 ro m a n Wr z a l I k ,6 a n d an a T o l y G. Gu r B a n o v 3 1Faculty of Earth Sciences, Department of Geochemistry, Mineralogy and Petrography, University of Silesia, Będzińska 60, 41-200 Sosnowiec, Poland 2Mineralogical Crystallography, Institute of Geological Sciences, University of Bern, Freiestrasse 3, CH-3012 Bern, Switzerland 3Institute of Geology of Ore Deposits, Geochemistry, Mineralogy and Petrography (IGEM) RAS, Staromonetny 35, 119017 Moscow, Russia 4OOO NPP TEPLOCHIM, Dmitrovskoye av. 71, 127238 Moscow, Russia 5Institute of Geochemistry, Mineralogy and Petrology, University of Warsaw, al. Żwirki i Wigury 93, 02-089 Warszawa, Poland 6Institute of Physics, University of Silesia, Uniwersytecka 4, 40-007 Katowice, Poland aB s T r a c T Kumtyubeite, Ca5(SiO4)2F2—the fluorine analog of reinhardbraunsite with a chondrodite-type structure—is a rock-forming mineral found in skarn carbonate-xenoliths in ignimbrites of the Upper Chegem volcanic structure, Kabardino-Balkaria, Northern Caucasus, Russia. -

Leucophoenicite Mn (Sio4)3(OH)2
2+ Leucophoenicite Mn7 (SiO4)3(OH)2 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Monoclinic. Point Group: 2=m: Crystals rare, typically slender, prismatic, elongated and striated [010], to 8 mm; in isolated grains or granular massive. Twinning: On k 001 , common, contact or interpenetrant twins, lamellar. f g Physical Properties: Cleavage: 001 , imperfect. Tenacity: Brittle. Hardness = 5.5{6 f g D(meas.) = 3.848 D(calc.) = [4.01] Optical Properties: Transparent to translucent. Color: Brown to light purple-red, raspberry-red, deep pink to light pink; rose-red to colorless in thin section. Luster: Vitreous. Optical Class: Biaxial ({). Pleochroism: Faint; rose-red 001 ; colorless 001 . Orientation: k f g ? f g X 001 cleavage. Dispersion: r > v; slight. ® = 1.751(3) ¯ = 1.771(3) ° = 1.782(3) ? f g 2V(meas.) = 74(5)± Cell Data: Space Group: P 21=a: a = 10.842(19) b = 4.826(6) c = 11.324(9) ¯ = 103:93(9)± Z = [2] X-ray Powder Pattern: Franklin, New Jersey, USA. 1.8063 (10), 2.877 (9), 2.684 (8), 4.36 (5), 3.612 (5), 2.365 (5), 2.620 (4) Chemistry: (1) (2) (3) (1) (2) (3) SiO2 26.36 26.7 26.7 CaO 5.67 2.4 2.8 FeO trace 0.3 0.3 Na2O 0.39 MnO 60.63 62.8 64.7 K2O 0.24 ZnO 3.87 0.0 0.0 H2O 2.64 [2.3] [2.8] MgO 0.21 5.5 2.7 Total 100.01 [100.0] [100.0] (1) Franklin, New Jersey, USA; composite of two analyses, corresponding to (Mn5:89Ca0:70Zn0:32 Na0:04Mg0:03K0:01)§=6:99(Si1:01O4)3(OH)2: (2) Kombat mine, Namibia; by electron microprobe, H2O by di®erence; corresponding to (Mn5:98Mg0:92Ca0:29Fe0:02)§=7:21(SiO4)3(OH)1:72: (3) Valsesia-Valtournanche area, Italy; by electron microprobe, H2O by di®erence; corresponding to (Mn6:16Mg0:45Ca0:34Fe0:03)§=6:98(SiO4)3(OH)2:10: Mineral Group: Leucophoenicite group. -

TEPHROITE from FRANKLIN, NEW JERSEY* Connbrrus S. Hunrsur
THE AMERICAN MINERALOGIST, VOL 46, MAY_JUNE, 1961 TEPHROITE FROM FRANKLIN, NEW JERSEY* ConNBrrus S. Hunrsur, Jn., Departmentof Mineralogy, Harvard, Uniaersity. Assrnlcr A study of tephroite specimens from Franklin and Sterling Hill, New Jersey showed in all of them the presence of thin sheets of willemite believed to be a product of exsolution. .fhese sheets are oriented parallel to the {100} and [010] planes of tephroite with the o and r axes of tephroite and willemite parallel. It is believed that Iittle zinc remains in the tephroite structure and that much of it reported in chemical analyses has been con- tributed by intergrown u'illemite. This conclusion is supported by experiments syn- thesizing tephroite. The indices of refraction and d spacing of {130} vary as would be expected with changes in amounts of MgO, FeO and CaO. INrnooucrroN 'fephroite, Mn2SiO4,a member of the olivine group, was describedas a new mineral from SterlingHill by Breithaupt in 1823.A chemicalanal- ysis of the original material was published by Brush (1864) together with severaladditional chemical analysesof tephroite made by others. These analysesreport ZnO in varying amounts which Brush attributed to invariably associatedzincite. Palache (1937) did not agree with Brush and stated " . that the molecularratios in someanalyses more nearly satisfy the orthosilicateformula when zinc is regardedas essen- tially a part of the mineral rather than as a constituent of mechanical inclusions." The present study was undertaken for the purpose of in- vestigatingthe variations in the propertiesof tephroite with changesin chemical composition, particularly the effect of zinc. Relationships were not expectedto be simplefor analysesshow, in addition to ZnO, variable amounts of MgO, FeO, and CaO. -
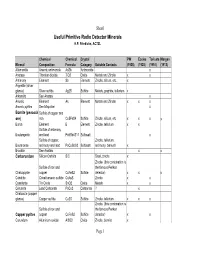
Useful Primitive Radio Detector Minerals H.P
Sheet1 Useful Primitive Radio Detector Minerals H.P. Friedichs, AC7ZL Chemical Chemical Crystal PW Eccles Toricata Morgan Mineral Composition Formula Category Suitable Contacts (1925) (1928) (1910) (1913) Allemontite Arsenic antimonide AsSb Antimonide x Anatase Titanium dioxide TiO2 Oxide Metals and Zincite x x Antimony Element Sb Element Zincite, silicon, etc. x Argentite (silver glance) Silver sulfide Ag2S Sulfide Metals, graphite, tellurium x Arkansite See Anatase x Arsenic Element As Element Metals and Zincite x x x Arsenic pyrites See Mispickel x Bornite (peacock Sulfide of copper and ore) iron Cu5FeS4 Sulfide Zincite, silicon, etc. x x x x Boron Element B Element Zincite, tellurium x x Sulfide of antimony Boulangerite and lead Pb5Sb4S11 Sulfosalt x Sulfide of copper, Zincite, tellurium, Bourmonite antimony and lead PbCuSbS3 Sulfosalt antimony, bismuth x Brookite See Anatase x x Carborundum Silicon Carbide SiC Steel, zincite x Zincite (this combination is Sulfide of iron and the famous Perikon Chalcopyrite copper CuFeS2 Sulfide detector) x x x Cobaltite Cobalt arsenic sulfide CoAsS Zincite x x Cassiterite Tin Oxide SnO2 Oxide Metals x x Cerussite Lead Carbonate PbCo3 Carbonate ?x Chalcocite (copper glance) Copper sulfide Cu2S Sulfide Zincite, tellurium x x x Zincite (this combination is Sulfide of iron and the famous Perikon Copper pyrites copper CuFeS2 Sulfide detector) x x Corundum Aluminum oxidde Al2O3 Oxide Zincite, bornite x Page 1 Sheet1 Covellite Copper sulfide CuS Sulfide Zincite, etc x x Cuprite (Cuprous oxide) Copper oxide -

Base Your Answers to Questions 1 and 2 on the Diagram Below, Which Shows the Results of Three Different Physical Tests, A, B, and C, That Were Performed on a Mineral
Base your answers to questions 1 and 2 on the diagram below, which shows the results of three different physical tests, A, B, and C, that were performed on a mineral. 1. The luster of this mineral could be determined by A) using an electronic balance B) using a graduated cylinder C) observing how light reflects from the surface of the mineral D) observing what happens when acid is placed on the mineral 2. Which mineral was tested? A) amphibole B) quartz C) galena D) graphite 3. Different arrangements of tetrahedra in the silicate group of minerals result in differences in the minerals' A) age, density, and smoothness B) cleavage, color, and abundance C) hardness, cleavage, and crystal shape D) chemical composition, size, and origin 4. The diagram of Bowen's Reaction Series below indicates the relative temperatures at which specific minerals crystallize as magma cools. Which statement is best supported by Bowen's Reaction Series? A) Most minerals crystallize at the same temperature. B) Most felsic minerals usually crystallize before most mafic minerals. C) Muscovite mica and quartz are the last minerals to crystallize as magma cools. D) Biotite mica is the first mineral to crystallize as magma cools. Base your answers to questions 5 through 8 on the 6. Moh's scale arranges minerals according to their data table below. relative A) resistance to breaking B) resistance to scratching C) specific heat D) specific gravity 7. Which statement is best supported by the data shown? A) An iron nail contains fluorite. B) A streak plate is composed of quartz. -

Jerrygibbsite Mn (Sio4)
2+ Jerrygibbsite Mn9 (SiO4)4(OH)2 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Orthorhombic. Point Group: mm2 (probable), or 2=m 2=m 2=m: As interlocking anhedral crystals, to 2 mm. Physical Properties: Cleavage: Imperfect on 001 . Hardness = 5.5 D(meas.) = 4.00(2) D(calc.) = 4.045 f g » Optical Properties: Transparent and translucent lamellae alternating 001 . Color: Violet-pink, with a brownish tinge; light pink in thin section. Strekakf: Light pink. Luster: Vitreous. Optical Class: Biaxial ({). Orientation: X = b; Y = c; Z = a. Dispersion: r > v; moderate. ® = 1.772(4) ¯ = 1.783(4) ° = 1.789(4) 2V(meas.) = 72± Cell Data: Space Group: P bn21 (probable), or P bnm: a = 4.875(2) b = 10.709(6) c = 28.18(2) Z = 4 X-ray Powder Pattern: Franklin, New Jersey, USA. 2.557 (100), 1.806 (100), 2.869 (78), 2.752 (49), 2.702 (46), 2.362 (39), 2.661 (34) Chemistry: (1) SiO2 27.1 FeO 0.3 MnO 64.1 ZnO 3.9 MgO 1.4 CaO 0.4 F 0.0 H2O 2.13 Total 99.3 (1) Franklin, New Jersey, USA; by electron microprobe, H2O by the Pen¯eld method; corresponds to (Mn7:86Zn0:59Mg0:24Ca0:16Fe0:14)§=8:99(SiO4)4(OH)2: Polymorphism & Series: Dimorphous with sonolite. Mineral Group: Leucophoenicite group. Occurrence: In a metamorphosed stratiform Zn-Mn deposit. Association: Franklinite, willemite, zincite, sonolite, leucophoenicite, tephroite. Distribution: From Franklin, Sussex Co., New Jersey, USA. Name: In honor of Professor Gerald V. Gibbs, Virginia Polytechnic Institute and State University, Blacksburg, Virginia, USA.