Allometric and Phylogenetic Variation in Insect Phosphorus 18, 103–109 Content
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insect Orders V: Panorpida & Hymenoptera
Insect Orders V: Panorpida & Hymenoptera • The Panorpida contain 5 orders: the Mecoptera, Siphonaptera, Diptera, Trichoptera and Lepidoptera. • Available evidence clearly indicates that the Lepidoptera and the Trichoptera are sister groups. • The Siphonaptera and Mecoptera are also closely related but it is not clear whether the Siponaptera is the sister group of all of the Mecoptera or a group (Boreidae) within the Mecoptera. If the latter is true, then the Mecoptera is paraphyletic as currently defined. • The Diptera is the sister group of the Siphonaptera + Mecoptera and together make up the Mecopteroids. • The Hymenoptera does not appear to be closely related to any of the other holometabolous orders. Mecoptera (Scorpionflies, hangingflies) • Classification. 600 species worldwide, arranged into 9 families (5 in the US). A very old group, many fossils from the Permian (260 mya) onward. • Structure. Most distinctive feature is the elongated clypeus and labrum that together form a rostrum. The order gets its common name from the gential segment of the male in the family Panorpodiae, which is bulbous and often curved forward above the abdomen, like the sting of a scorpion. Larvae are caterpillar-like or grub- like. • Natural history. Scorpionflies are most common in cool, moist habitats. They get the name “hangingflies” from their habit of hanging upside down on vegetation. Larvae and adult males are mostly predators or scavengers. Adult females are usually scavengers. Larvae and adults in some groups may feed on vegetation. Larvae of most species are terrestrial and caterpillar-like in body form. Larvae of some species are aquatic. In the family Bittacidae males attract females for mating by releasing a sex pheromone and then presenting the female with a nuptial gift. -
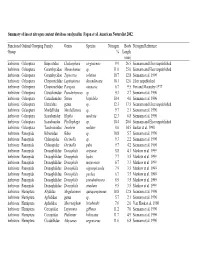
Summary of Insect Nitrogen Content Database Analyzed in Fagan Et Al
Summary of insect nitrogen content database analyzed in Fagan et al. American Naturalist 2002. Functional Ordinal Grouping Family Genus Species Nitrogen Body Nitrogen Reference Group % Length (mm) herbivore Coleoptera Buprestidae Chalcophora virginiensis 9.9 26.5 Siemann and Elser unpublished herbivore Coleoptera Cerambycidae Monochamus sp. 11.0 25.0 Siemann and Elser unpublished herbivore Coleoptera Cerambycidae Typocerus velutina 10.7 12.8 Siemann et al. 1996 herbivore Coleoptera Chrysomelidae Leptinotarsa decemlineata 10.1 12.0 Elser unpublished herbivore Coleoptera Chrysomelidae Paropsis atomaria 6.7 9.5 Fox and Macauley 1977 herbivore Coleoptera Curculionidae Pseudorhyncus sp. 9.3 2.7 Siemann et al. 1996 herbivore Coleoptera Curculionidae Sitona hispidula 10.4 4.0 Siemann et al. 1996 herbivore Coleoptera Elateridae genus sp. 12.3 17.5 Siemann and Elser unpublished herbivore Coleoptera Mordellidae Mordellistena sp. 9.7 2.1 Siemann et al. 1996 herbivore Coleoptera Scarabaeidae Hoplia modesta 12.3 6.8 Siemann et al. 1996 herbivore Coleoptera Scarabaeidae Phyllophaga sp. 10.4 20.0 Siemann and Elser unpublished herbivore Coleoptera Tenebrionidae Tenebrio molitor 8.0 14.5 Barker et al. 1998 herbivore Panorpida Bibionidae Bibio sp. 10.8 5.7 Siemann et al. 1996 herbivore Panorpida Chloropidae Oscinella sp. 9.3 2.2 Siemann et al. 1996 herbivore Panorpida Chloropidae Oscinella pube 9.7 4.2 Siemann et al. 1996 herbivore Panorpida Drosophilidae Drosophila arizonae 8.8 4.1 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila hydei 7.7 3.5 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila mojavensis 6.7 3.3 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila nigrospiracula 7.9 3.5 Markow et al. -

Fleas (Siphonaptera) Are Cretaceous, and Evolved with Theria
bioRxiv preprint doi: https://doi.org/10.1101/014308; this version posted January 24, 2015. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Fleas (Siphonaptera) are Cretaceous, and Evolved with Theria Qiyun Zhu1, Michael Hastriter2, Michael Whiting2, 3, Katharina Dittmar1, 4* Jan. 23, 2015 Abstract: Fleas (order Siphonaptera) are highly-specialized, diverse blood-feeding ectoparasites of mammals and birds with an enigmatic evolutionary history and obscure origin. We here present a molecular phylogenetic study based on a compre- hensive taxon sampling of 259 flea taxa, representing 16 of the 18 extant families of this order. A Bayesian phylogenetic tree with strong nodal support was recovered, consisting of seven sequentially derived lineages with Macropsyllidae at the base and Stephanocircidae as the second basal group. Divergence times of flea lineages were estimated based on fossil records and host specific associations to bats (Chiroptera), showing that the common ancestor of extant Siphonaptera split from its clos- est mecopteran sister group in the Early Cretaceous and basal lineages diversified during the Late Cretaceous. However, most of the intraordinal divergence into families took place after the K-Pg boundary. Ancestral states of host association and bioge- ographical distribution were reconstructed, suggesting with high likelihood that fleas originated in the southern continents (Gondwana) and migrated from South America to their extant distributions in a relatively short time frame. Theria (placental mammals and marsupials) represent the most likely ancestral host group of extant Siphonaptera, with marsupials occupying a more important role than previously assumed. -

Phylogeny of Endopterygote Insects, the Most Successful Lineage of Living Organisms*
REVIEW Eur. J. Entomol. 96: 237-253, 1999 ISSN 1210-5759 Phylogeny of endopterygote insects, the most successful lineage of living organisms* N iels P. KRISTENSEN Zoological Museum, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen 0, Denmark; e-mail: [email protected] Key words. Insecta, Endopterygota, Holometabola, phylogeny, diversification modes, Megaloptera, Raphidioptera, Neuroptera, Coleóptera, Strepsiptera, Díptera, Mecoptera, Siphonaptera, Trichoptera, Lepidoptera, Hymenoptera Abstract. The monophyly of the Endopterygota is supported primarily by the specialized larva without external wing buds and with degradable eyes, as well as by the quiescence of the last immature (pupal) stage; a specialized morphology of the latter is not an en dopterygote groundplan trait. There is weak support for the basal endopterygote splitting event being between a Neuropterida + Co leóptera clade and a Mecopterida + Hymenoptera clade; a fully sclerotized sitophore plate in the adult is a newly recognized possible groundplan autapomorphy of the latter. The molecular evidence for a Strepsiptera + Díptera clade is differently interpreted by advo cates of parsimony and maximum likelihood analyses of sequence data, and the morphological evidence for the monophyly of this clade is ambiguous. The basal diversification patterns within the principal endopterygote clades (“orders”) are succinctly reviewed. The truly species-rich clades are almost consistently quite subordinate. The identification of “key innovations” promoting evolution -

New Species of Neopanorpa (Mecoptera) from Vietnam, with a Key to the Species of Mecoptera of Vietnam Author(S): Wesley J
New Species of Neopanorpa (Mecoptera) from Vietnam, with a Key to the Species of Mecoptera of Vietnam Author(s): Wesley J. Bicha, Nathan Schiff, Thai Hong Pham, Aaron Lancaster and Brian Scheffler Source: Proceedings of the Entomological Society of Washington, 119(4):529-544. Published By: Entomological Society of Washington https://doi.org/10.4289/0013-8797.119.4.529 URL: http://www.bioone.org/doi/full/10.4289/0013-8797.119.4.529 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/ terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. PROC. ENTOMOL. SOC. WASH. 119(4), 2017, pp. 529–544 NEW SPECIES OF NEOPANORPA (MECOPTERA) FROM VIETNAM, WITH A KEY TO THE SPECIES OF MECOPTERA OF VIETNAM urn:lsid:zoobank.org:pub:CE24D561-5F0E-482F-953D-B0B809D4A607 WESLEY J. BICHA,NATHAN SCHIFF,THAI HONG PHAM,AARON LANCASTER, AND BRIAN SCHEFFLER (WJB) Tropical Research Associates Entomology, 521 46th Street, Sacramento, California 95819 (e-mail: [email protected]); (NS) United States Department of Agriculture Forest Service, Southern Research Station, Center for Bottomland Hardwoods Research, P.O. -

Evolution of the Insects
CY501-C11[407-467].qxd 3/2/05 12:56 PM Page 407 quark11 Quark11:Desktop Folder:CY501-Grimaldi:Quark_files: But, for the point of wisdom, I would choose to Know the mind that stirs Between the wings of Bees and building wasps. –George Eliot, The Spanish Gypsy 11HHymenoptera:ymenoptera: Ants, Bees, and Ants,Other Wasps Bees, and The order Hymenoptera comprises one of the four “hyperdi- various times between the Late Permian and Early Triassic. verse” insectO lineages;ther the others – Diptera, Lepidoptera, Wasps and, Thus, unlike some of the basal holometabolan orders, the of course, Coleoptera – are also holometabolous. Among Hymenoptera have a relatively recent origin, first appearing holometabolans, Hymenoptera is perhaps the most difficult in the Late Triassic. Since the Triassic, the Hymenoptera have to place in a phylogenetic framework, excepting the enig- truly come into their own, having radiated extensively in the matic twisted-wings, order Strepsiptera. Hymenoptera are Jurassic, again in the Cretaceous, and again (within certain morphologically isolated among orders of Holometabola, family-level lineages) during the Tertiary. The hymenopteran consisting of a complex mixture of primitive traits and bauplan, in both structure and function, has been tremen- numerous autapomorphies, leaving little evidence to which dously successful. group they are most closely related. Present evidence indi- While the beetles today boast the largest number of cates that the Holometabola can be organized into two major species among all orders, Hymenoptera may eventually rival lineages: the Coleoptera ϩ Neuropterida and the Panorpida. or even surpass the diversity of coleopterans (Kristensen, It is to the Panorpida that the Hymenoptera appear to be 1999a; Grissell, 1999). -

Fossil Calibrations for the Arthropod Tree of Life
bioRxiv preprint doi: https://doi.org/10.1101/044859; this version posted June 10, 2016. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. FOSSIL CALIBRATIONS FOR THE ARTHROPOD TREE OF LIFE AUTHORS Joanna M. Wolfe1*, Allison C. Daley2,3, David A. Legg3, Gregory D. Edgecombe4 1 Department of Earth, Atmospheric & Planetary Sciences, Massachusetts Institute of Technology, Cambridge, MA 02139, USA 2 Department of Zoology, University of Oxford, South Parks Road, Oxford OX1 3PS, UK 3 Oxford University Museum of Natural History, Parks Road, Oxford OX1 3PZ, UK 4 Department of Earth Sciences, The Natural History Museum, Cromwell Road, London SW7 5BD, UK *Corresponding author: [email protected] ABSTRACT Fossil age data and molecular sequences are increasingly combined to establish a timescale for the Tree of Life. Arthropods, as the most species-rich and morphologically disparate animal phylum, have received substantial attention, particularly with regard to questions such as the timing of habitat shifts (e.g. terrestrialisation), genome evolution (e.g. gene family duplication and functional evolution), origins of novel characters and behaviours (e.g. wings and flight, venom, silk), biogeography, rate of diversification (e.g. Cambrian explosion, insect coevolution with angiosperms, evolution of crab body plans), and the evolution of arthropod microbiomes. We present herein a series of rigorously vetted calibration fossils for arthropod evolutionary history, taking into account recently published guidelines for best practice in fossil calibration. -

Florida Scorpionfly, Panorpa Floridana Byers (Insecta: Mecoptera: Panorpidae) 1
EENY-538 Florida Scorpionfly, Panorpa floridana Byers (Insecta: Mecoptera: Panorpidae) 1 Louis A. Somma and James C. Dunford2 Introduction 2013). However, rather than being rare, this species is prob- ably especially cryptic and difficult to locate in the wild. The order Mecoptera, often called the scorpionflies, is a relatively small order of insects that contains nine families and is distributed worldwide (Somma et al. 2014). Mecopterans, along with the orders that include the fleas (Siphonaptera), true flies (Diptera), caddisflies (Tricoph- tera) and moths and butterflies (Lepidoptera), comprise the group Panorpida (Grimaldi and Engel 2005). Panorpida is an important evolutionary grouping of insects within the holometabolous orders (those that have a larval and pupal stages, rather than nymphs) fundamentally different from the beetles (Coleoptera) and the ants, bees, and wasps (Hymenoptera) (Grimaldi and Engel 2005). Within this group is the Antliophora, a monophyletic (arising from Figure 1. Male Florida scorpionfly, Panorpa floridana Byers, a single ancestor) grouping of Mecoptera, Diptera, and photographed in Gold Head Branch Ravine, Clay County, Florida. Siphonaptera, suggesting that the scorpionflies are most Credits: Stephen Cresswell (photographic voucher FNAI Reference closely related to true flies and fleas (Grimaldi and Engel Code: N13CRE01FLUS, Reference ID: 189911; MorphoBank M152670) 2005, Somma et al. 2014). Distribution The Florida scorpionfly, Panorpa floridana Byers, is a To date, Panorpa floridana is known only from specimens little-known insect, endemic to northern peninsular collected from Alachua and Clay counties in northern Florida (Byers 1993; Somma and Dunford 2008). The first peninsular Florida. The two specimens from Alachua description of this species of scorpionfly (Panorpidae) in County are the holotype, a male collected in Gainesville 1993 was limited to five specimens, the last one collected in near the San Felasco Hammock in 1970 (currently housed 1982 (Byers 1993; Somma and Dunford 2008). -

ENTOMOLOGY 322 LAB 20 Female Reproductive System
ENTOMOLOGY 322 LAB 20 Female Reproductive System The female reproductive system consists of paired ovaries (Ov) each with multiple ovarioles (Ovl) Egg maturation occurs gradually as each oocyte passes downward along the ovariole. Oocytes are first formed via meiosis in the germarium (Grm), and progress downward through the vitellarium (Vtl), where the yolk proteins are added to the developing oocyte. The most mature oocytes are located at the bottom of each ovariole, where they are retained until laying. As you might expect, the number of ovarioles is closely related to the rate of egg production. The typical pattern is 4, 6, or 8 ovarioles/ovary, but may be as many as 100-200 ovarioles/ovary occurs in Diptera and eusocial Hymenoptera, and termite queens many have up to 2400 ovarioles/ovary. Each ovary is connected to a lateral oviduct (Odl), and the paired lateral oviducts meet medially in the common oviduct (Odc). Female insects can store sperm for long periods of time (years) in the spermatheca (Spt), an outgrowth of the common oviduct that is commonly associated with a spermathecal gland (SptGl). Accessory glands (AcGl) may also be present. These glands perform a variety of functions, including producing secretions which hold eggs together in a cluster or glue eggs to substrate, producing the egg case or ootheca in some Orthoptera, providing larval nutrition (as milk glands) in viviparous Diptera (e.g., Glossina), and serving as venom glands in aculeate Hymenoptera. Figure 20.1 (Snodgrass, 1935) The location of the female gonopore varies among the insects. Primitively, the gonopore is located behind the 7th sternum. -

(Insecta: Papilionidea = Mecopteroidea) and the Origin Of
Paleontological Journal, Vol. 38, Suppl. 2, 2004, pp. S204–S213. Original Russian Text Copyright © 2004 by Novokshonov. English Translation Copyright © 2004 by MAIK “Nauka /Interperiodica” (Russia). The First Mecopteroids (Insecta: Papilionidea = Mecopteroidea) and the Origin of Scorpionflies (Panorpida = Mecoptera), with Description of a Legless Eruciform Larva from the Lower Permian of Tshekarda V. G. Novokshonov† Perm State University, ul. Genkelya 1, Perm, 614005 Russia Received July 10, 2002 Abstract—The origin of insects of the mecopteroid complex and the early evolution of true scorpionflies are discussed. A legless eruciform larva, Curvilarva tshekardensis sp. nov., is described from the Lower Permian of Tshekarda. INTRODUCTION SEPARATION OF MECOPTEROIDS Among modern fauna, the superorder Papilionidea The first mecopteroids are close to neuropteroids represents one of the largest groups of holometabolous (Myrmeleontidea = Neuropteroidea) and originate insects, being slightly exceeded in terms of number of from primitive members of the latter superorder (Mar- species only by beetles and hymenopterans. The earli- tynova, 1957; Rasnitsyn, 1980b) the larvae of which est mecopteroids are known from the Early Permian had not yet become specialized in predatory feeding. and are represented by Trichoptera and Panorpida The earliest neuropteroids entered the fossil record (= Mecoptera) (Tillyard, 1926; Martynov, 1940; Suka- simultaneously with mecopteroids. tcheva, 1976; Kukalová-Peck and Willmann, 1990). We will compare only those characters of the first The rest of the mecopteroid orders have not been mecopteroids and neuropteroids that are visible in the recorded from the Paleozoic, except for presumed and Permian fossils. highly questionable remains of Diptera that have been True neuropterans appear to be most similar to the found in the Permian continental deposits of France earliest mecopteroids in wing structure. -

American Museum Novitates
AMERICAN MUSEUM NOVITATES Number 3793, 23 pp. January 6, 2014 The long-tongued Cretaceous scorpionfly Parapolycentropus Grimaldi and Rasnitsyn (Mecoptera: Pseudopolycentropodidae): New data and interpretations DAVID GRIMALDI1 AND M. AndrEW JOHNSTON1,2 ABSTRACT The genus Parapolycentropus, originally described for two species in 99 myo Burmese amber, is unique among Mecoptera for its long, thin proboscis and possession of just the mesothoracic pair of wings. A new series of 19 specimens with excellent preservation allows description and redescription of virtually all morphological details. Male terminalia are very similar to those of the Holarctic Recent family of “snow fleas,” the Boreidae. Thoracic sclerites are highly convergent with nematocerous Diptera in the expansion of the mesothorax and great reduction of the pro- and metathoraces. The metathoracic wing vestige appears to be just the tegula; axial sclerites are lost. Details of the pretarsal claws are described; in P. paraburmiticus Grimaldi and Rasnitsyn the outer claw of the meso- and metathoracic pretarsi is elongate and the inner claw reduced. The proboscis is comprised not of a labial tube and “pseudolabellum” (contra Ren et al., 2009), but is mostly maxillary in origin, with the outer valves probably being galeae and the central, serrated stylet probably the hypopharynx. Abdominal sternites are greatly reduced (more so in females), suggesting that the abdomen was distensible, a feature that is common in some fluid-feeding insects. The proboscis, claw, and sternite modifications indicate that Parapolycentropus fed on the hemolymph of small insects, not the blood of vertebrates. Key Words: Myanmar, amber, mouthparts, predation, hematophagy. 1 Division of Invertebrate Zoology, American Museum of Natural History, Central Park West at 79th St., New York, New York 10024-5192. -
The Biology of the Mecoptera the BIOLOGY of the MECOPTERA FRANK V[. CARPENTER* Morals of the Hymenoptera. Our National Parks
The Biology of the Mecoptera 41 THE BIOLOGY OF THE MECOPTERA FRANK V[. CARPENTER* Among the curious phenomena of nature none is more unfortunate than the close correlation which exists between the number of species in an insect order and the number of entomologists concerned with it. The entomological world is filled with students investigating the classification, economies, society, pathology, eugenics, intelligence and morals of the Hymenoptera. Our National Parks and Forests are menaced annually by coleopterists, destroying the natural geology and tearing the barks from the trees in their search for new species. The Lepidoptera, espe- cially the more attractive groups, are gradually becoming extinct, because of the enthusiastic quest for aberrations and queer varieties. But the smaller orders of insects are rarely the object of biological or taxonomic investigation. A list of the neuropterists in all the world would not equal a list of the coleopterists in some of our larger cities. This evening I propose to discuss the habits and life- history of one of the smallest and therefore most neglected orders of insects--the Mecoptera, or scorpion-flies. Many of you have probably never seen one of these insects out- side a museum, for they are very seclusive. They fly seldom and for short distances only, and usually keep close to the ground. When disturbed they either dart to the ground and hide among the dead leaves, or they fly to the under sur- faces of the leaves and branches of shrubs, where they can be seen only with difficulty. So far as humans are con- cerned they.