Implications for Phylogenetic Analysis of EF-La Sequences in Insects
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Moths from Texas (Noctuidae, Tortricidae)
1968 Joltrnal of the Lepidopterists' Societu 133 NEW MOTHS FROM TEXAS (NOCTUIDAE, TORTRICIDAE) ANDRE BLANCHARD 3023 Underwood, Houston, Texas As a retirement hobby, I decided, six years ago, to catalog the moths of Texas. My wife and I have been collecting moths all over Texas for the last four years. As the work progressed, I came to realize that I may have to settle, more modestly, for a "Contributions Toward A Catalog of the Moths of Texas." The number of species in my collection which had apparently never been taken in Texas is quite large, and the number of those which seem to be new to science, particularly from the mountain ranges and desert areas of West Texas, is much larger than I ever expected. I have been fortunate in interesting several specialists in describing some of these new species: Dr. C. L. Hogue (196S), Mr. McElvare ( 1966), Dr. E. L. Todd (1966) have described three. Difficult cases are now and, in the future, will be submitted to experts. I have de scribed the male of a fourth species (1966), the female of which was described by Dr. F. H. Hindge (1966). While getting more material for my intcnded catalog, I shall describe as many of the new species as I can name without becoming guilty of adding to the confusion which already exists in some genera. In the present paper, I describe six new noctuids and one tortricid species. All types were collected by A. &. M. E. Blanchard. Acronicta vallis cola Blanchard, new species (PI. I, fig. J; PI. -

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

Natural Communities of Michigan: Classification and Description
Natural Communities of Michigan: Classification and Description Prepared by: Michael A. Kost, Dennis A. Albert, Joshua G. Cohen, Bradford S. Slaughter, Rebecca K. Schillo, Christopher R. Weber, and Kim A. Chapman Michigan Natural Features Inventory P.O. Box 13036 Lansing, MI 48901-3036 For: Michigan Department of Natural Resources Wildlife Division and Forest, Mineral and Fire Management Division September 30, 2007 Report Number 2007-21 Version 1.2 Last Updated: July 9, 2010 Suggested Citation: Kost, M.A., D.A. Albert, J.G. Cohen, B.S. Slaughter, R.K. Schillo, C.R. Weber, and K.A. Chapman. 2007. Natural Communities of Michigan: Classification and Description. Michigan Natural Features Inventory, Report Number 2007-21, Lansing, MI. 314 pp. Copyright 2007 Michigan State University Board of Trustees. Michigan State University Extension programs and materials are open to all without regard to race, color, national origin, gender, religion, age, disability, political beliefs, sexual orientation, marital status or family status. Cover photos: Top left, Dry Sand Prairie at Indian Lake, Newaygo County (M. Kost); top right, Limestone Bedrock Lakeshore, Summer Island, Delta County (J. Cohen); lower left, Muskeg, Luce County (J. Cohen); and lower right, Mesic Northern Forest as a matrix natural community, Porcupine Mountains Wilderness State Park, Ontonagon County (M. Kost). Acknowledgements We thank the Michigan Department of Natural Resources Wildlife Division and Forest, Mineral, and Fire Management Division for funding this effort to classify and describe the natural communities of Michigan. This work relied heavily on data collected by many present and former Michigan Natural Features Inventory (MNFI) field scientists and collaborators, including members of the Michigan Natural Areas Council. -

Insect Orders V: Panorpida & Hymenoptera
Insect Orders V: Panorpida & Hymenoptera • The Panorpida contain 5 orders: the Mecoptera, Siphonaptera, Diptera, Trichoptera and Lepidoptera. • Available evidence clearly indicates that the Lepidoptera and the Trichoptera are sister groups. • The Siphonaptera and Mecoptera are also closely related but it is not clear whether the Siponaptera is the sister group of all of the Mecoptera or a group (Boreidae) within the Mecoptera. If the latter is true, then the Mecoptera is paraphyletic as currently defined. • The Diptera is the sister group of the Siphonaptera + Mecoptera and together make up the Mecopteroids. • The Hymenoptera does not appear to be closely related to any of the other holometabolous orders. Mecoptera (Scorpionflies, hangingflies) • Classification. 600 species worldwide, arranged into 9 families (5 in the US). A very old group, many fossils from the Permian (260 mya) onward. • Structure. Most distinctive feature is the elongated clypeus and labrum that together form a rostrum. The order gets its common name from the gential segment of the male in the family Panorpodiae, which is bulbous and often curved forward above the abdomen, like the sting of a scorpion. Larvae are caterpillar-like or grub- like. • Natural history. Scorpionflies are most common in cool, moist habitats. They get the name “hangingflies” from their habit of hanging upside down on vegetation. Larvae and adult males are mostly predators or scavengers. Adult females are usually scavengers. Larvae and adults in some groups may feed on vegetation. Larvae of most species are terrestrial and caterpillar-like in body form. Larvae of some species are aquatic. In the family Bittacidae males attract females for mating by releasing a sex pheromone and then presenting the female with a nuptial gift. -
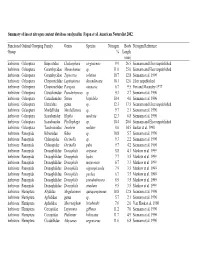
Summary of Insect Nitrogen Content Database Analyzed in Fagan Et Al
Summary of insect nitrogen content database analyzed in Fagan et al. American Naturalist 2002. Functional Ordinal Grouping Family Genus Species Nitrogen Body Nitrogen Reference Group % Length (mm) herbivore Coleoptera Buprestidae Chalcophora virginiensis 9.9 26.5 Siemann and Elser unpublished herbivore Coleoptera Cerambycidae Monochamus sp. 11.0 25.0 Siemann and Elser unpublished herbivore Coleoptera Cerambycidae Typocerus velutina 10.7 12.8 Siemann et al. 1996 herbivore Coleoptera Chrysomelidae Leptinotarsa decemlineata 10.1 12.0 Elser unpublished herbivore Coleoptera Chrysomelidae Paropsis atomaria 6.7 9.5 Fox and Macauley 1977 herbivore Coleoptera Curculionidae Pseudorhyncus sp. 9.3 2.7 Siemann et al. 1996 herbivore Coleoptera Curculionidae Sitona hispidula 10.4 4.0 Siemann et al. 1996 herbivore Coleoptera Elateridae genus sp. 12.3 17.5 Siemann and Elser unpublished herbivore Coleoptera Mordellidae Mordellistena sp. 9.7 2.1 Siemann et al. 1996 herbivore Coleoptera Scarabaeidae Hoplia modesta 12.3 6.8 Siemann et al. 1996 herbivore Coleoptera Scarabaeidae Phyllophaga sp. 10.4 20.0 Siemann and Elser unpublished herbivore Coleoptera Tenebrionidae Tenebrio molitor 8.0 14.5 Barker et al. 1998 herbivore Panorpida Bibionidae Bibio sp. 10.8 5.7 Siemann et al. 1996 herbivore Panorpida Chloropidae Oscinella sp. 9.3 2.2 Siemann et al. 1996 herbivore Panorpida Chloropidae Oscinella pube 9.7 4.2 Siemann et al. 1996 herbivore Panorpida Drosophilidae Drosophila arizonae 8.8 4.1 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila hydei 7.7 3.5 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila mojavensis 6.7 3.3 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila nigrospiracula 7.9 3.5 Markow et al. -

Fleas (Siphonaptera) Are Cretaceous, and Evolved with Theria
bioRxiv preprint doi: https://doi.org/10.1101/014308; this version posted January 24, 2015. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Fleas (Siphonaptera) are Cretaceous, and Evolved with Theria Qiyun Zhu1, Michael Hastriter2, Michael Whiting2, 3, Katharina Dittmar1, 4* Jan. 23, 2015 Abstract: Fleas (order Siphonaptera) are highly-specialized, diverse blood-feeding ectoparasites of mammals and birds with an enigmatic evolutionary history and obscure origin. We here present a molecular phylogenetic study based on a compre- hensive taxon sampling of 259 flea taxa, representing 16 of the 18 extant families of this order. A Bayesian phylogenetic tree with strong nodal support was recovered, consisting of seven sequentially derived lineages with Macropsyllidae at the base and Stephanocircidae as the second basal group. Divergence times of flea lineages were estimated based on fossil records and host specific associations to bats (Chiroptera), showing that the common ancestor of extant Siphonaptera split from its clos- est mecopteran sister group in the Early Cretaceous and basal lineages diversified during the Late Cretaceous. However, most of the intraordinal divergence into families took place after the K-Pg boundary. Ancestral states of host association and bioge- ographical distribution were reconstructed, suggesting with high likelihood that fleas originated in the southern continents (Gondwana) and migrated from South America to their extant distributions in a relatively short time frame. Theria (placental mammals and marsupials) represent the most likely ancestral host group of extant Siphonaptera, with marsupials occupying a more important role than previously assumed. -

Allometric and Phylogenetic Variation in Insect Phosphorus 18, 103–109 Content
Functional Blackwell Publishing, Ltd. Ecology 2004 Allometric and phylogenetic variation in insect phosphorus 18, 103–109 content H. A. WOODS,*† W. F. FAGAN,‡ J. J. ELSER§ and J. F. HARRISON§ *Section of Integrative Biology C0930, University of Texas at Austin, Austin, TX 78712, USA, ‡Department of Biology, University of Maryland, College Park, MD 20742, USA, and §Department of Biology, Arizona State University, Tempe, AZ 85287–1501, USA Summary 1. Phosphorus content was measured in adult insects and arachnids from 170 species collected in the Sonoran Desert. 2. Across insect body sizes spanning four orders of magnitude, phosphorus content was inversely related to body mass. The largest species (∼1 g dry) had phosphorus contents that were only about 60% (0·62% P absolute) as high as phosphorus contents of the smallest species (∼0·0001 g dry; 0·97% P). Negative phosphorus allometry was observed within each of seven insect orders and within arachnids. 3. Phosphorus contents of insect predators and herbivores were statistically indistinguishable. 4. More recently derived orders tended to have lower phosphorus contents – with the exception of the most recently derived group (Panorpida = Diptera + Lepidoptera), which had high phosphorus contents. Key-words: Allometry, body size, exoskeleton, phosphorus limitation, stoichiometry Functional Ecology (2004) 18, 103–109 processes dependent on elemental composition (e.g. Introduction nutrient cycling) and because P occupies a key position Herbivorous insects face a fundamental asymmetry in the recently developed theory of ecological stoichio- between the nutrient contents of their body tissues and metry (Sterner & Elser 2002). those of the plants they eat (Slansky & Feeny 1977; Several expectations can be derived a priori by McNeill & Southwood 1978; Mattson 1980; Strong, extensions of previous work on N. -

Phylogeny of Endopterygote Insects, the Most Successful Lineage of Living Organisms*
REVIEW Eur. J. Entomol. 96: 237-253, 1999 ISSN 1210-5759 Phylogeny of endopterygote insects, the most successful lineage of living organisms* N iels P. KRISTENSEN Zoological Museum, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen 0, Denmark; e-mail: [email protected] Key words. Insecta, Endopterygota, Holometabola, phylogeny, diversification modes, Megaloptera, Raphidioptera, Neuroptera, Coleóptera, Strepsiptera, Díptera, Mecoptera, Siphonaptera, Trichoptera, Lepidoptera, Hymenoptera Abstract. The monophyly of the Endopterygota is supported primarily by the specialized larva without external wing buds and with degradable eyes, as well as by the quiescence of the last immature (pupal) stage; a specialized morphology of the latter is not an en dopterygote groundplan trait. There is weak support for the basal endopterygote splitting event being between a Neuropterida + Co leóptera clade and a Mecopterida + Hymenoptera clade; a fully sclerotized sitophore plate in the adult is a newly recognized possible groundplan autapomorphy of the latter. The molecular evidence for a Strepsiptera + Díptera clade is differently interpreted by advo cates of parsimony and maximum likelihood analyses of sequence data, and the morphological evidence for the monophyly of this clade is ambiguous. The basal diversification patterns within the principal endopterygote clades (“orders”) are succinctly reviewed. The truly species-rich clades are almost consistently quite subordinate. The identification of “key innovations” promoting evolution -

Merrimac Farm WMA Insect List As of September 2014 Order Family
Merrimac Farm WMA Insect List as of September 2014 Order Family Common Name Scientific Name Acari Ixodidae American Dog Tick Dermacentor variabilis Araneae Anyphaenidae Ghost Spider Hibana sp. Araneae Araneidae Larinia directa Larinia directa Araneae Araneidae Star-bellied Orbweaver Acanthepeira stellata Araneae Araneidae White Micrathena Micrathena mitrata Araneae Araneidae Spined Micrathena Micrathena gracilis Araneae Lycosidae Wolf Spider Hogna sp. Araneae Lycosidae Thin-legged Wolf Spider Pardosa sp. Araneae Lycosidae Rabid Wolf Spider Rabidosa rabida Araneae Oxyopidae Lynx Spider Oxyopes aglossus Araneae Salticidae Jumping Spider Pelegrina proterva? Araneae Salticidae Jumping Spider Phidippus princeps Araneae Salticidae Jumping Spider Tutellina elegans Araneae Salticidae Peppered Jumper Pelegrina galathea Araneae Thomisidae Northern Crab Spider Mecaphesa asperata Araneae Thomisidae Swift Crab Spider Mecaphesa celer Araneae Thomisidae White-banded Crab Spider Misumenoides formosipes Blattodea Cryptocercidae Brown-hooded Cockroach Cryptocercus punctulatus Coleoptera Cantharidae Margined Leatherwing Chauliognathus marginatus Coleoptera Cantharidae Soldier Beetle Podabrus rugosulus Coleoptera Carabidae Vivid Metallic Ground Beetle Chlaenius sp. Coleoptera Carabidae Vivid Metallic Ground Beetle Chlaenius emarginatus Coleoptera Carabidae Six-spotted Tiger Beetle Cicindela sexguttata Coleoptera Cerambycidae Flower Longhorn Beetle Strangalia luteicornis Coleoptera Cerambycidae Locust Borer Megacyllene robiniae Coleoptera Cerambycidae Red -

Moths of North Carolina - Early Draft 1
Noctuidae Basilodes pepita Gold Moth 10 9 8 n=3 • • • 7 High Mt. • • • • 6 • N 5 • u 4 3 • • • m 2 • • b 1 • e 0 • • r 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 NC counties: 17 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec o 10 f 9 n=22 = Sighting or Collection 8 • 7 Low Mt. High counts of: in NC since 2001 F 6 l 5 3 - Swain - 2001-08-15 = Not seen since 2001 4 • i 3 2 - Swain - 2001-08-18 g 2 Status Rank h 1 1 - Ashe - 2000-07-30 0 NC US NC Global t 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 D Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec a 10 10 9 9 t 8 n=13 8 n=5 e 7 Pd 7 CP s 6 6 5 5 4 4 3 3 2 2 1 1 0 0 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Three periods to each month: 1-10 / 11-20 / 21-31 FAMILY: Noctuidae SUBFAMILY: Amphipyrinae TRIBE: Stiriini TAXONOMIC_COMMENTS: A very distinct genus of 7 species found from Costa Rica into Canada. -

New Species of Neopanorpa (Mecoptera) from Vietnam, with a Key to the Species of Mecoptera of Vietnam Author(S): Wesley J
New Species of Neopanorpa (Mecoptera) from Vietnam, with a Key to the Species of Mecoptera of Vietnam Author(s): Wesley J. Bicha, Nathan Schiff, Thai Hong Pham, Aaron Lancaster and Brian Scheffler Source: Proceedings of the Entomological Society of Washington, 119(4):529-544. Published By: Entomological Society of Washington https://doi.org/10.4289/0013-8797.119.4.529 URL: http://www.bioone.org/doi/full/10.4289/0013-8797.119.4.529 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/ terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. PROC. ENTOMOL. SOC. WASH. 119(4), 2017, pp. 529–544 NEW SPECIES OF NEOPANORPA (MECOPTERA) FROM VIETNAM, WITH A KEY TO THE SPECIES OF MECOPTERA OF VIETNAM urn:lsid:zoobank.org:pub:CE24D561-5F0E-482F-953D-B0B809D4A607 WESLEY J. BICHA,NATHAN SCHIFF,THAI HONG PHAM,AARON LANCASTER, AND BRIAN SCHEFFLER (WJB) Tropical Research Associates Entomology, 521 46th Street, Sacramento, California 95819 (e-mail: [email protected]); (NS) United States Department of Agriculture Forest Service, Southern Research Station, Center for Bottomland Hardwoods Research, P.O. -

Evolution of the Insects
CY501-C11[407-467].qxd 3/2/05 12:56 PM Page 407 quark11 Quark11:Desktop Folder:CY501-Grimaldi:Quark_files: But, for the point of wisdom, I would choose to Know the mind that stirs Between the wings of Bees and building wasps. –George Eliot, The Spanish Gypsy 11HHymenoptera:ymenoptera: Ants, Bees, and Ants,Other Wasps Bees, and The order Hymenoptera comprises one of the four “hyperdi- various times between the Late Permian and Early Triassic. verse” insectO lineages;ther the others – Diptera, Lepidoptera, Wasps and, Thus, unlike some of the basal holometabolan orders, the of course, Coleoptera – are also holometabolous. Among Hymenoptera have a relatively recent origin, first appearing holometabolans, Hymenoptera is perhaps the most difficult in the Late Triassic. Since the Triassic, the Hymenoptera have to place in a phylogenetic framework, excepting the enig- truly come into their own, having radiated extensively in the matic twisted-wings, order Strepsiptera. Hymenoptera are Jurassic, again in the Cretaceous, and again (within certain morphologically isolated among orders of Holometabola, family-level lineages) during the Tertiary. The hymenopteran consisting of a complex mixture of primitive traits and bauplan, in both structure and function, has been tremen- numerous autapomorphies, leaving little evidence to which dously successful. group they are most closely related. Present evidence indi- While the beetles today boast the largest number of cates that the Holometabola can be organized into two major species among all orders, Hymenoptera may eventually rival lineages: the Coleoptera ϩ Neuropterida and the Panorpida. or even surpass the diversity of coleopterans (Kristensen, It is to the Panorpida that the Hymenoptera appear to be 1999a; Grissell, 1999).