Insect Orders V: Panorpida & Hymenoptera
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
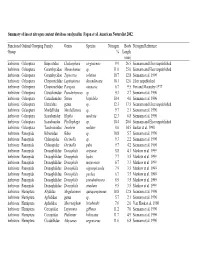
Summary of Insect Nitrogen Content Database Analyzed in Fagan Et Al
Summary of insect nitrogen content database analyzed in Fagan et al. American Naturalist 2002. Functional Ordinal Grouping Family Genus Species Nitrogen Body Nitrogen Reference Group % Length (mm) herbivore Coleoptera Buprestidae Chalcophora virginiensis 9.9 26.5 Siemann and Elser unpublished herbivore Coleoptera Cerambycidae Monochamus sp. 11.0 25.0 Siemann and Elser unpublished herbivore Coleoptera Cerambycidae Typocerus velutina 10.7 12.8 Siemann et al. 1996 herbivore Coleoptera Chrysomelidae Leptinotarsa decemlineata 10.1 12.0 Elser unpublished herbivore Coleoptera Chrysomelidae Paropsis atomaria 6.7 9.5 Fox and Macauley 1977 herbivore Coleoptera Curculionidae Pseudorhyncus sp. 9.3 2.7 Siemann et al. 1996 herbivore Coleoptera Curculionidae Sitona hispidula 10.4 4.0 Siemann et al. 1996 herbivore Coleoptera Elateridae genus sp. 12.3 17.5 Siemann and Elser unpublished herbivore Coleoptera Mordellidae Mordellistena sp. 9.7 2.1 Siemann et al. 1996 herbivore Coleoptera Scarabaeidae Hoplia modesta 12.3 6.8 Siemann et al. 1996 herbivore Coleoptera Scarabaeidae Phyllophaga sp. 10.4 20.0 Siemann and Elser unpublished herbivore Coleoptera Tenebrionidae Tenebrio molitor 8.0 14.5 Barker et al. 1998 herbivore Panorpida Bibionidae Bibio sp. 10.8 5.7 Siemann et al. 1996 herbivore Panorpida Chloropidae Oscinella sp. 9.3 2.2 Siemann et al. 1996 herbivore Panorpida Chloropidae Oscinella pube 9.7 4.2 Siemann et al. 1996 herbivore Panorpida Drosophilidae Drosophila arizonae 8.8 4.1 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila hydei 7.7 3.5 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila mojavensis 6.7 3.3 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila nigrospiracula 7.9 3.5 Markow et al. -

Huchard Et Al., 2006 1.Pdf
Acetylcholinesterase genes within the Diptera: takeover and loss in true flies Elise Huchard, Michel Martinez, Haoues Alout, Emmanuel Douzery, Georges Lutfalla, Arnaud Berthomieu, Claire Berticat, Michel Raymond, Mylene Weill To cite this version: Elise Huchard, Michel Martinez, Haoues Alout, Emmanuel Douzery, Georges Lutfalla, et al.. Acetyl- cholinesterase genes within the Diptera: takeover and loss in true flies. Proceedings of the Royal Society B: Biological Sciences, Royal Society, The, 2006, 273 (1601), pp.2595-2604. 10.1098/rspb.2006.3621. hal-01945529 HAL Id: hal-01945529 https://hal.archives-ouvertes.fr/hal-01945529 Submitted on 29 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Proc. R. Soc. B (2006) 273, 2595–2604 doi:10.1098/rspb.2006.3621 Published online 18 July 2006 Acetylcholinesterase genes within the Diptera: takeover and loss in true flies Elise Huchard1, Michel Martinez2, Haoues Alout1, Emmanuel J. P. Douzery1, Georges Lutfalla3, Arnaud Berthomieu1, Claire Berticat1, Michel Raymond1,* and Myle`ne Weill1 1Institut des Sciences -

Table of Contents 2
Southwest Association of Freshwater Invertebrate Taxonomists (SAFIT) List of Freshwater Macroinvertebrate Taxa from California and Adjacent States including Standard Taxonomic Effort Levels 1 March 2011 Austin Brady Richards and D. Christopher Rogers Table of Contents 2 1.0 Introduction 4 1.1 Acknowledgments 5 2.0 Standard Taxonomic Effort 5 2.1 Rules for Developing a Standard Taxonomic Effort Document 5 2.2 Changes from the Previous Version 6 2.3 The SAFIT Standard Taxonomic List 6 3.0 Methods and Materials 7 3.1 Habitat information 7 3.2 Geographic Scope 7 3.3 Abbreviations used in the STE List 8 3.4 Life Stage Terminology 8 4.0 Rare, Threatened and Endangered Species 8 5.0 Literature Cited 9 Appendix I. The SAFIT Standard Taxonomic Effort List 10 Phylum Silicea 11 Phylum Cnidaria 12 Phylum Platyhelminthes 14 Phylum Nemertea 15 Phylum Nemata 16 Phylum Nematomorpha 17 Phylum Entoprocta 18 Phylum Ectoprocta 19 Phylum Mollusca 20 Phylum Annelida 32 Class Hirudinea Class Branchiobdella Class Polychaeta Class Oligochaeta Phylum Arthropoda Subphylum Chelicerata, Subclass Acari 35 Subphylum Crustacea 47 Subphylum Hexapoda Class Collembola 69 Class Insecta Order Ephemeroptera 71 Order Odonata 95 Order Plecoptera 112 Order Hemiptera 126 Order Megaloptera 139 Order Neuroptera 141 Order Trichoptera 143 Order Lepidoptera 165 2 Order Coleoptera 167 Order Diptera 219 3 1.0 Introduction The Southwest Association of Freshwater Invertebrate Taxonomists (SAFIT) is charged through its charter to develop standardized levels for the taxonomic identification of aquatic macroinvertebrates in support of bioassessment. This document defines the standard levels of taxonomic effort (STE) for bioassessment data compatible with the Surface Water Ambient Monitoring Program (SWAMP) bioassessment protocols (Ode, 2007) or similar procedures. -

A New Synonymy in the Horsefly Genus Hybomitra (Diptera: Tabanidae)
a new synonymy in the horsefly genus HYBOMITRA (diptera: tabanidae) Theo Zeegers The taxonomy and nomenclature of Hybomitra solstitialis is critically revised. Based on a re-examination of the type, H. solstitialis is found to be identical with the species currently known as H. ciureai syn. nov. Since the 1950s the name H. solstitalis has been misinterpreted in European literature. This concept, introduced by Lyneborg, is shown to be a light coloured variety of H. bimaculata. introduction The horseflies (family Tabanidae) are a median The discovery by Lyneborg (15) of the impor- sized family (about 170 species in Europe, Chvála tance of the shape of the subgenital plate, espe- et al. (172)) of median to very large flies (6- cially in the H. bimaculata-group, was a major 25 mm). Females are notorious for sucking blood breakthrough in the taxonomy of the genus on both humans and livestock. In the palearctic Hybomitra. Unfortunately, he distinguished too region, the tribe Tabanini of the subfamily Taban- many species due to underestimating of the inae is the most abundant group of horseflies. colour variation. This was largely corrected by In the palearctic region the horsefly genera are Chvála et al. (172), introducing a system that has well defined. Identification of species is often been followed since (Moucha 176, Olsufjev 177, difficult due to variability in coloration and Trojan 17, Timmer 10, Chvála 1, Zeegers paucity of structural features. Identification is & Van Haaren 2000, Stubbs & Drake 2001, especially challenging in the genus Hybomitra Portillo 2002). Enderlein, 122, characterized by the presence of an ocellar tubercle on the vertex. -

Species-Specific Behavioral Differences in Tsetse Fly Genital
Chapter 15 Species-Specific Behavioral Differences in Tsetse Fly Genital Morphology and Probable Cryptic Female Choice R.D. Briceño and W.G. Eberhard Abstract A long-standing mystery in morphological evolution is why male geni- talia tend to diverge more rapidly than other structures. One possible explana- tion of this trend is that male genitalia function as “internal courtship devices,” and are under sexual selection by cryptic female choice (CFC) to induce female responses that improve the male’s chances of fathering her offspring. Males of closely related species, which have species-specific genital structures, are thought to provide divergent stimulation. Testing this hypothesis has been difficult; the pre- sumed genital courtship behavior is hidden from view inside the female; appropri- ate experimental manipulations of male and female genitalia are often technically difficult and seldom performed; and most studies of how the male’s genitalia inter- act with those of the female are limited to a single species in a given group, thus limiting opportunities for comparisons of species-specific structures. In this chap- ter, we summarize data from morphological, behavioral, and experimental studies of six species in the tsetse fly genus Glossina, including new X-ray recordings that allowed visualization of events inside the female during real time. Species-specific male genital structures perform dramatic, stereotyped, rhythmic movements, some on the external surface of the female’s abdomen and others within her reproduc- tive tract. Counting conservatively, a female Glossina may sense stimuli from the male’s genitalia at up to 8 sites on her body during some stages of copulation. -

Insecta Diptera) in Freshwater (Excluding Simulidae, Culicidae, Chironomidae, Tipulidae and Tabanidae) Rüdiger Wagner University of Kassel
Entomology Publications Entomology 2008 Global diversity of dipteran families (Insecta Diptera) in freshwater (excluding Simulidae, Culicidae, Chironomidae, Tipulidae and Tabanidae) Rüdiger Wagner University of Kassel Miroslav Barták Czech University of Agriculture Art Borkent Salmon Arm Gregory W. Courtney Iowa State University, [email protected] Follow this and additional works at: http://lib.dr.iastate.edu/ent_pubs BoudewPart ofijn the GoBddeeiodivrisersity Commons, Biology Commons, Entomology Commons, and the TRoyerarle Bestrlgiialan a Indnstit Aquaute of Nticat uErcaol Scienlogyce Cs ommons TheSee nex tompc page forle addte bitioniblaiol agruthorapshic information for this item can be found at http://lib.dr.iastate.edu/ ent_pubs/41. For information on how to cite this item, please visit http://lib.dr.iastate.edu/ howtocite.html. This Book Chapter is brought to you for free and open access by the Entomology at Iowa State University Digital Repository. It has been accepted for inclusion in Entomology Publications by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Global diversity of dipteran families (Insecta Diptera) in freshwater (excluding Simulidae, Culicidae, Chironomidae, Tipulidae and Tabanidae) Abstract Today’s knowledge of worldwide species diversity of 19 families of aquatic Diptera in Continental Waters is presented. Nevertheless, we have to face for certain in most groups a restricted knowledge about distribution, ecology and systematic, -

Insect Parasitoids
Insect Parasitoids Parasitoid—An animal that feeds in or on another living animal for a relavely long 4me, consuming all or most of its 4ssues and eventually killing it. Insect parasitoids account for 7% of described species. Most parasitoids are found in 3 orders: Hymenoptera (76%) 1 evolu4onary lineage Diptera (22%) 21 evolu4onary lineages Coleoptera (2%) 14 evolu4onary lineages Parasitoids of Ants Family Species Order (Tribe) Richness Natural History Strepsiptera Myrmecolacidae ~100 Males are parasitoids of adult worker ants; females are parasitoids of mantids and orthopterans Hymenoptera Braconidae 35 Parasitoids of adult worker ants (Neoneurinae) Chalicididae 6 Parasitoids of worker larvae-pupae (Smicromorphinae) Diapriidae >25 Parasitoids of worker larvae-pupae Eucharitidae ~500 Parasitoids of worker larvae-pupae Ichneumonidae 16 Parasitoids of worker larvae-pupae (Paxylommatinae) Diptera Phoridae ~900 Parasitoids of adult worker ants Tachinidae 1 Endoparasitoid of founding queens of Lasius Parasitoids of Ants Family Species Order (Tribe) Richness Natural History Strepsiptera Myrmecolacidae ~100 Males are parasitoids of adult worker ants; females are parasitoids of mantids and orthopterans Hymenoptera Braconidae 35 Parasitoids of adult worker ants (Neoneurinae) Chalicididae 6 Parasitoids of worker larvae-pupae (Smicromorphinae) Diapriidae >10 Parasitoids of worker larvae-pupae Eucharitidae ~500 Parasitoids of worker larvae-pupae Ichneumonidae 16 Parasitoids of worker larvae-pupae (Paxylommatinae) Diptera Phoridae ~900 Parasitoids -

Flies) Benjamin Kongyeli Badii
Chapter Phylogeny and Functional Morphology of Diptera (Flies) Benjamin Kongyeli Badii Abstract The order Diptera includes all true flies. Members of this order are the most ecologically diverse and probably have a greater economic impact on humans than any other group of insects. The application of explicit methods of phylogenetic and morphological analysis has revealed weaknesses in the traditional classification of dipteran insects, but little progress has been made to achieve a robust, stable clas- sification that reflects evolutionary relationships and morphological adaptations for a more precise understanding of their developmental biology and behavioral ecol- ogy. The current status of Diptera phylogenetics is reviewed in this chapter. Also, key aspects of the morphology of the different life stages of the flies, particularly characters useful for taxonomic purposes and for an understanding of the group’s biology have been described with an emphasis on newer contributions and progress in understanding this important group of insects. Keywords: Tephritoidea, Diptera flies, Nematocera, Brachycera metamorphosis, larva 1. Introduction Phylogeny refers to the evolutionary history of a taxonomic group of organisms. Phylogeny is essential in understanding the biodiversity, genetics, evolution, and ecology among groups of organisms [1, 2]. Functional morphology involves the study of the relationships between the structure of an organism and the function of the various parts of an organism. The old adage “form follows function” is a guiding principle of functional morphology. It helps in understanding the ways in which body structures can be used to produce a wide variety of different behaviors, including moving, feeding, fighting, and reproducing. It thus, integrates concepts from physiology, evolution, anatomy and development, and synthesizes the diverse ways that biological and physical factors interact in the lives of organisms [3]. -

Fleas (Siphonaptera) Are Cretaceous, and Evolved with Theria
bioRxiv preprint doi: https://doi.org/10.1101/014308; this version posted January 24, 2015. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Fleas (Siphonaptera) are Cretaceous, and Evolved with Theria Qiyun Zhu1, Michael Hastriter2, Michael Whiting2, 3, Katharina Dittmar1, 4* Jan. 23, 2015 Abstract: Fleas (order Siphonaptera) are highly-specialized, diverse blood-feeding ectoparasites of mammals and birds with an enigmatic evolutionary history and obscure origin. We here present a molecular phylogenetic study based on a compre- hensive taxon sampling of 259 flea taxa, representing 16 of the 18 extant families of this order. A Bayesian phylogenetic tree with strong nodal support was recovered, consisting of seven sequentially derived lineages with Macropsyllidae at the base and Stephanocircidae as the second basal group. Divergence times of flea lineages were estimated based on fossil records and host specific associations to bats (Chiroptera), showing that the common ancestor of extant Siphonaptera split from its clos- est mecopteran sister group in the Early Cretaceous and basal lineages diversified during the Late Cretaceous. However, most of the intraordinal divergence into families took place after the K-Pg boundary. Ancestral states of host association and bioge- ographical distribution were reconstructed, suggesting with high likelihood that fleas originated in the southern continents (Gondwana) and migrated from South America to their extant distributions in a relatively short time frame. Theria (placental mammals and marsupials) represent the most likely ancestral host group of extant Siphonaptera, with marsupials occupying a more important role than previously assumed. -

Allometric and Phylogenetic Variation in Insect Phosphorus 18, 103–109 Content
Functional Blackwell Publishing, Ltd. Ecology 2004 Allometric and phylogenetic variation in insect phosphorus 18, 103–109 content H. A. WOODS,*† W. F. FAGAN,‡ J. J. ELSER§ and J. F. HARRISON§ *Section of Integrative Biology C0930, University of Texas at Austin, Austin, TX 78712, USA, ‡Department of Biology, University of Maryland, College Park, MD 20742, USA, and §Department of Biology, Arizona State University, Tempe, AZ 85287–1501, USA Summary 1. Phosphorus content was measured in adult insects and arachnids from 170 species collected in the Sonoran Desert. 2. Across insect body sizes spanning four orders of magnitude, phosphorus content was inversely related to body mass. The largest species (∼1 g dry) had phosphorus contents that were only about 60% (0·62% P absolute) as high as phosphorus contents of the smallest species (∼0·0001 g dry; 0·97% P). Negative phosphorus allometry was observed within each of seven insect orders and within arachnids. 3. Phosphorus contents of insect predators and herbivores were statistically indistinguishable. 4. More recently derived orders tended to have lower phosphorus contents – with the exception of the most recently derived group (Panorpida = Diptera + Lepidoptera), which had high phosphorus contents. Key-words: Allometry, body size, exoskeleton, phosphorus limitation, stoichiometry Functional Ecology (2004) 18, 103–109 processes dependent on elemental composition (e.g. Introduction nutrient cycling) and because P occupies a key position Herbivorous insects face a fundamental asymmetry in the recently developed theory of ecological stoichio- between the nutrient contents of their body tissues and metry (Sterner & Elser 2002). those of the plants they eat (Slansky & Feeny 1977; Several expectations can be derived a priori by McNeill & Southwood 1978; Mattson 1980; Strong, extensions of previous work on N. -

Phylogeny of Endopterygote Insects, the Most Successful Lineage of Living Organisms*
REVIEW Eur. J. Entomol. 96: 237-253, 1999 ISSN 1210-5759 Phylogeny of endopterygote insects, the most successful lineage of living organisms* N iels P. KRISTENSEN Zoological Museum, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen 0, Denmark; e-mail: [email protected] Key words. Insecta, Endopterygota, Holometabola, phylogeny, diversification modes, Megaloptera, Raphidioptera, Neuroptera, Coleóptera, Strepsiptera, Díptera, Mecoptera, Siphonaptera, Trichoptera, Lepidoptera, Hymenoptera Abstract. The monophyly of the Endopterygota is supported primarily by the specialized larva without external wing buds and with degradable eyes, as well as by the quiescence of the last immature (pupal) stage; a specialized morphology of the latter is not an en dopterygote groundplan trait. There is weak support for the basal endopterygote splitting event being between a Neuropterida + Co leóptera clade and a Mecopterida + Hymenoptera clade; a fully sclerotized sitophore plate in the adult is a newly recognized possible groundplan autapomorphy of the latter. The molecular evidence for a Strepsiptera + Díptera clade is differently interpreted by advo cates of parsimony and maximum likelihood analyses of sequence data, and the morphological evidence for the monophyly of this clade is ambiguous. The basal diversification patterns within the principal endopterygote clades (“orders”) are succinctly reviewed. The truly species-rich clades are almost consistently quite subordinate. The identification of “key innovations” promoting evolution -

Diptera) in East Africa
A peer-reviewed open-access journal ZooKeys 769: 117–144Morphological (2018) re-description and molecular identification of Tabanidae... 117 doi: 10.3897/zookeys.769.21144 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Morphological re-description and molecular identification of Tabanidae (Diptera) in East Africa Claire M. Mugasa1,2, Jandouwe Villinger1, Joseph Gitau1, Nelly Ndungu1,3, Marc Ciosi1,4, Daniel Masiga1 1 International Centre of Insect Physiology and Ecology (ICIPE), Nairobi, Kenya 2 School of Biosecurity Biotechnical Laboratory Sciences, College of Veterinary Medicine, Animal Resources and Biosecurity (COVAB), Makerere University, Kampala, Uganda 3 Social Insects Research Group, Department of Zoology and Entomo- logy, University of Pretoria, Hatfield, 0028 Pretoria, South Africa 4 Institute of Molecular Cell and Systems Biology, University of Glasgow, Glasgow, UK Corresponding author: Daniel Masiga ([email protected]) Academic editor: T. Dikow | Received 19 October 2017 | Accepted 9 April 2018 | Published 26 June 2018 http://zoobank.org/AB4EED07-0C95-4020-B4BB-E6EEE5AC8D02 Citation: Mugasa CM, Villinger J, Gitau J, Ndungu N, Ciosi M, Masiga D (2018) Morphological re-description and molecular identification of Tabanidae (Diptera) in East Africa. ZooKeys 769: 117–144.https://doi.org/10.3897/ zookeys.769.21144 Abstract Biting flies of the family Tabanidae are important vectors of human and animal diseases across conti- nents. However, records of Africa tabanids are fragmentary and mostly cursory. To improve identification, documentation and description of Tabanidae in East Africa, a baseline survey for the identification and description of Tabanidae in three eastern African countries was conducted. Tabanids from various loca- tions in Uganda (Wakiso District), Tanzania (Tarangire National Park) and Kenya (Shimba Hills National Reserve, Muhaka, Nguruman) were collected.