Fleas (Siphonaptera) Are Cretaceous, and Evolved with Theria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NEWS 84June 2019
flea NEWS 84 June 2019 http://esanetworks.org/ FLEA NEWS is a twice-yearly newsletter about fleas (Siphonaptera). Recipients are urged to check any citations given here before including them in publications. Many of our sources are abstracting journals and current literature sources, and citations have not necessarily been checked for accuracy or consistent formatting. Recipients are urged to contribute items of interest to the profession for inclusion herein, including: Flea research citations from journals that are not indexed, Announcements and Requests for material, Contact information for a Directory of Siphonapterists (name, mailing address, email address, and areas of interest - Systematics, Ecology, Control, etc.), Abstracts of research planned or in progress, Book and Literature Reviews, Biography, Hypotheses, and Anecdotes. Send to: R. L. Bossard, Ph.D. Editor, Flea News [email protected] Organizers of the Flea News Network are Drs. R. L. Bossard and N. C. Hinkle. N. C. Hinkle, Ph.D. Dept. of Entomology Univ. of Georgia Athens GA 30602-2603 USA [email protected] (706) 583-8043 Assistant Editor J. R. Kucera, M.S. Contents Editorial Announcements Featured research Directory of Siphonapterists Editorial Dear Flea News Reader, The current Flea News is abbreviated so the Siphonaptera Literature list can appear in its entirety in the next Flea News. The change will consolidate all of 2019 citations in one Flea News. From The Lancet (Kneebone, 2019): "A collaboration with Erica McAlister, an entomologist at the Natural History Museum in London, UK, has revealed interesting parallels. As Senior Curator for Diptera and Siphonaptera, Erica is responsible for over a million two-winged insects in the museum's collection and she is a master at identification. -

Volume 41, 2000
BAT RESEARCH NEWS Volume 41 : No. 1 Spring 2000 I I BAT RESEARCH NEWS Volume 41: Numbers 1–4 2000 Original Issues Compiled by Dr. G. Roy Horst, Publisher and Managing Editor of Bat Research News, 2000. Copyright 2011 Bat Research News. All rights reserved. This material is protected by copyright and may not be reproduced, transmitted, posted on a Web site or a listserve, or disseminated in any form or by any means without prior written permission from the Publisher, Dr. Margaret A. Griffiths. The material is for individual use only. Bat Research News is ISSN # 0005-6227. BAT RESEARCH NEWS Volume41 Spring 2000 Numberl Contents Resolution on Rabies Exposure Merlin Tuttle and Thomas Griffiths o o o o eo o o o • o o o o o o o o o o o o o o o o 0 o o o o o o o o o o o 0 o o o 1 E - Mail Directory - 2000 Compiled by Roy Horst •••• 0 ...................... 0 ••••••••••••••••••••••• 2 ,t:.'. Recent Literature Compiled by :Margaret Griffiths . : ....••... •"r''• ..., .... >.•••••• , ••••• • ••< ...... 19 ,.!,..j,..,' ""o: ,II ,' f 'lf.,·,,- .,'b'l: ,~··.,., lfl!t • 0'( Titles Presented at the 7th Bat Researc:b Confei'ebee~;Moscow :i'\prill4-16~ '1999,., ..,, ~ .• , ' ' • I"',.., .. ' ""!' ,. Compiled by Roy Horst .. : .......... ~ ... ~· ....... : :· ,"'·~ .• ~:• .... ; •. ,·~ •.•, .. , ........ 22 ·.t.'t, J .,•• ~~ Letters to the Editor 26 I ••• 0 ••••• 0 •••••••••••• 0 ••••••• 0. 0. 0 0 ••••••• 0 •• 0. 0 •••••••• 0 ••••••••• 30 News . " Future Meetings, Conferences and Symposium ..................... ~ ..,•'.: .. ,. ·..; .... 31 Front Cover The illustration of Rhinolophus ferrumequinum on the front cover of this issue is by Philippe Penicaud . from his very handsome series of drawings representing the bats of France. -

Insect Orders V: Panorpida & Hymenoptera
Insect Orders V: Panorpida & Hymenoptera • The Panorpida contain 5 orders: the Mecoptera, Siphonaptera, Diptera, Trichoptera and Lepidoptera. • Available evidence clearly indicates that the Lepidoptera and the Trichoptera are sister groups. • The Siphonaptera and Mecoptera are also closely related but it is not clear whether the Siponaptera is the sister group of all of the Mecoptera or a group (Boreidae) within the Mecoptera. If the latter is true, then the Mecoptera is paraphyletic as currently defined. • The Diptera is the sister group of the Siphonaptera + Mecoptera and together make up the Mecopteroids. • The Hymenoptera does not appear to be closely related to any of the other holometabolous orders. Mecoptera (Scorpionflies, hangingflies) • Classification. 600 species worldwide, arranged into 9 families (5 in the US). A very old group, many fossils from the Permian (260 mya) onward. • Structure. Most distinctive feature is the elongated clypeus and labrum that together form a rostrum. The order gets its common name from the gential segment of the male in the family Panorpodiae, which is bulbous and often curved forward above the abdomen, like the sting of a scorpion. Larvae are caterpillar-like or grub- like. • Natural history. Scorpionflies are most common in cool, moist habitats. They get the name “hangingflies” from their habit of hanging upside down on vegetation. Larvae and adult males are mostly predators or scavengers. Adult females are usually scavengers. Larvae and adults in some groups may feed on vegetation. Larvae of most species are terrestrial and caterpillar-like in body form. Larvae of some species are aquatic. In the family Bittacidae males attract females for mating by releasing a sex pheromone and then presenting the female with a nuptial gift. -

Fleas, Hosts and Habitat: What Can We Predict About the Spread of Vector-Borne Zoonotic Diseases?
2010 Fleas, Hosts and Habitat: What can we predict about the spread of vector-borne zoonotic diseases? Ph.D. Dissertation Megan M. Friggens School of Forestry I I I \, l " FLEAS, HOSTS AND HABITAT: WHAT CAN WE PREDICT ABOUT THE SPREAD OF VECTOR-BORNE ZOONOTIC DISEASES? by Megan M. Friggens A Dissertation Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Forest Science Northern Arizona University May 2010 ?Jii@~-~-u-_- Robert R. Parmenter, Ph. D. ~",l(*~ l.~ Paulette L. Ford, Ph. D. --=z:r-J'l1jU~ David M. Wagner, Ph. D. ABSTRACT FLEAS, HOSTS AND HABITAT: WHAT CAN WE PREDICT ABOUT THE SPREAD OF VECTOR-BORNE ZOONOTIC DISEASES? MEGAN M. FRIGGENS Vector-borne diseases of humans and wildlife are experiencing resurgence across the globe. I examine the dynamics of flea borne diseases through a comparative analysis of flea literature and analyses of field data collected from three sites in New Mexico: The Sevilleta National Wildlife Refuge, the Sandia Mountains and the Valles Caldera National Preserve (VCNP). My objectives were to use these analyses to better predict and manage for the spread of diseases such as plague (Yersinia pestis). To assess the impact of anthropogenic disturbance on flea communities, I compiled and analyzed data from 63 published empirical studies. Anthropogenic disturbance is associated with conditions conducive to increased transmission of flea-borne diseases. Most measures of flea infestation increased with increasing disturbance or peaked at intermediate levels of disturbance. Future trends of habitat and climate change will probably favor the spread of flea-borne disease. -

Fleas and Flea-Borne Diseases
International Journal of Infectious Diseases 14 (2010) e667–e676 Contents lists available at ScienceDirect International Journal of Infectious Diseases journal homepage: www.elsevier.com/locate/ijid Review Fleas and flea-borne diseases Idir Bitam a, Katharina Dittmar b, Philippe Parola a, Michael F. Whiting c, Didier Raoult a,* a Unite´ de Recherche en Maladies Infectieuses Tropicales Emergentes, CNRS-IRD UMR 6236, Faculte´ de Me´decine, Universite´ de la Me´diterrane´e, 27 Bd Jean Moulin, 13385 Marseille Cedex 5, France b Department of Biological Sciences, SUNY at Buffalo, Buffalo, NY, USA c Department of Biology, Brigham Young University, Provo, Utah, USA ARTICLE INFO SUMMARY Article history: Flea-borne infections are emerging or re-emerging throughout the world, and their incidence is on the Received 3 February 2009 rise. Furthermore, their distribution and that of their vectors is shifting and expanding. This publication Received in revised form 2 June 2009 reviews general flea biology and the distribution of the flea-borne diseases of public health importance Accepted 4 November 2009 throughout the world, their principal flea vectors, and the extent of their public health burden. Such an Corresponding Editor: William Cameron, overall review is necessary to understand the importance of this group of infections and the resources Ottawa, Canada that must be allocated to their control by public health authorities to ensure their timely diagnosis and treatment. Keywords: ß 2010 International Society for Infectious Diseases. Published by Elsevier Ltd. All rights reserved. Flea Siphonaptera Plague Yersinia pestis Rickettsia Bartonella Introduction to 16 families and 238 genera have been described, but only a minority is synanthropic, that is they live in close association with The past decades have seen a dramatic change in the geographic humans (Table 1).4,5 and host ranges of many vector-borne pathogens, and their diseases. -

Flea NEWS 56 Department of Entomology Iowa State University, Ames, Iowa 50011 50, June, 1995; No
flea NEWS 56 Department of Entomology Iowa State University, Ames, Iowa 50011 50, June, 1995; No. 51, December, 1995; No. 52, June, 1996, No. 53, December, Table of Contents 1996; No. 54, June, 1997, 55, January, 1998 and this number. Literature..............................662 Mailing List Changes .............668 ❊❄❊❄❊❄❊ Miscellanea...........................660 MISCELLANEA Flea News (Online) has now been FLEA NEWS is a biannual newsletter assigned the following International devoted to matters involving insects Standard Serial Number: ISSN 1089- belonging to the order Siphonaptera (fleas) 7631 and related subjects. It is compiled and distributed free of charge by Robert E. Lewis ❖❏❖❏❖❏❖ <[email protected]> in cooperation with the Department of Entomology at Iowa State Dr. Glen Chilton of the Department University, Ames, IA, and a grant in aid of Biology, St. Mary's College, Calg- from Wellmark International. ary, Alberta, T2S 2N5, Canada, rec- Flea News is mainly bibliographic in nature. Many of the sources are abstracting ently called my attention to the Birds journals and title pages and not all citations of North America accounts published have been checked for completeness or jointly by the American Ornithol- accuracy. Additional information will be ogists' Union and the Academy of provided upon written or e-mail request. Natural Sciences, Philadelphia. To Further, recipients are urged to contribute date 320 accounts have been publish- items of interest to the professon for ed and the following titles include inclusion herein. information on fleas: This newsletter is now available in 7. Northern Mockingbird electronic format. The preferred method of 11. Tree Swallow accessing the electronic version is through the 12. -

BÖCEKLERİN SINIFLANDIRILMASI (Takım Düzeyinde)
BÖCEKLERİN SINIFLANDIRILMASI (TAKIM DÜZEYİNDE) GÖKHAN AYDIN 2016 Editör : Gökhan AYDIN Dizgi : Ziya ÖNCÜ ISBN : 978-605-87432-3-6 Böceklerin Sınıflandırılması isimli eğitim amaçlı hazırlanan bilgisayar programı için lütfen aşağıda verilen linki tıklayarak programı ücretsiz olarak bilgisayarınıza yükleyin. http://atabeymyo.sdu.edu.tr/assets/uploads/sites/76/files/siniflama-05102016.exe Eğitim Amaçlı Bilgisayar Programı ISBN: 978-605-87432-2-9 İçindekiler İçindekiler i Önsöz vi 1. Protura - Coneheads 1 1.1 Özellikleri 1 1.2 Ekonomik Önemi 2 1.3 Bunları Biliyor musunuz? 2 2. Collembola - Springtails 3 2.1 Özellikleri 3 2.2 Ekonomik Önemi 4 2.3 Bunları Biliyor musunuz? 4 3. Thysanura - Silverfish 6 3.1 Özellikleri 6 3.2 Ekonomik Önemi 7 3.3 Bunları Biliyor musunuz? 7 4. Microcoryphia - Bristletails 8 4.1 Özellikleri 8 4.2 Ekonomik Önemi 9 5. Diplura 10 5.1 Özellikleri 10 5.2 Ekonomik Önemi 10 5.3 Bunları Biliyor musunuz? 11 6. Plocoptera – Stoneflies 12 6.1 Özellikleri 12 6.2 Ekonomik Önemi 12 6.3 Bunları Biliyor musunuz? 13 7. Embioptera - webspinners 14 7.1 Özellikleri 15 7.2 Ekonomik Önemi 15 7.3 Bunları Biliyor musunuz? 15 8. Orthoptera–Grasshoppers, Crickets 16 8.1 Özellikleri 16 8.2 Ekonomik Önemi 16 8.3 Bunları Biliyor musunuz? 17 i 9. Phasmida - Walkingsticks 20 9.1 Özellikleri 20 9.2 Ekonomik Önemi 21 9.3 Bunları Biliyor musunuz? 21 10. Dermaptera - Earwigs 23 10.1 Özellikleri 23 10.2 Ekonomik Önemi 24 10.3 Bunları Biliyor musunuz? 24 11. Zoraptera 25 11.1 Özellikleri 25 11.2 Ekonomik Önemi 25 11.3 Bunları Biliyor musunuz? 26 12. -

Smithsonian Miscellaneous Collections
SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 104, NUMBER 7 THE FEEDING APPARATUS OF BITING AND SUCKING INSECTS AFFECTING MAN AND ANIMALS BY R. E. SNODGRASS Bureau of Entomology and Plant Quarantine Agricultural Research Administration U. S. Department of Agriculture (Publication 3773) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION OCTOBER 24, 1944 SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 104, NUMBER 7 THE FEEDING APPARATUS OF BITING AND SUCKING INSECTS AFFECTING MAN AND ANIMALS BY R. E. SNODGRASS Bureau of Entomology and Plant Quarantine Agricultural Research Administration U. S. Department of Agriculture P£R\ (Publication 3773) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION OCTOBER 24, 1944 BALTIMORE, MO., U. S. A. THE FEEDING APPARATUS OF BITING AND SUCKING INSECTS AFFECTING MAN AND ANIMALS By R. E. SNODGRASS Bureau of Entomology and Plant Quarantine, Agricultural Research Administration, U. S. Department of Agriculture CONTENTS Page Introduction 2 I. The cockroach. Order Orthoptera 3 The head and the organs of ingestion 4 General structure of the head and mouth parts 4 The labrum 7 The mandibles 8 The maxillae 10 The labium 13 The hypopharynx 14 The preoral food cavity 17 The mechanism of ingestion 18 The alimentary canal 19 II. The biting lice and booklice. Orders Mallophaga and Corrodentia. 21 III. The elephant louse 30 IV. The sucking lice. Order Anoplura 31 V. The flies. Order Diptera 36 Mosquitoes. Family Culicidae 37 Sand flies. Family Psychodidae 50 Biting midges. Family Heleidae 54 Black flies. Family Simuliidae 56 Net-winged midges. Family Blepharoceratidae 61 Horse flies. Family Tabanidae 61 Snipe flies. Family Rhagionidae 64 Robber flies. Family Asilidae 66 Special features of the Cyclorrhapha 68 Eye gnats. -
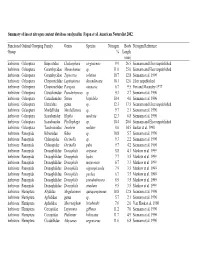
Summary of Insect Nitrogen Content Database Analyzed in Fagan Et Al
Summary of insect nitrogen content database analyzed in Fagan et al. American Naturalist 2002. Functional Ordinal Grouping Family Genus Species Nitrogen Body Nitrogen Reference Group % Length (mm) herbivore Coleoptera Buprestidae Chalcophora virginiensis 9.9 26.5 Siemann and Elser unpublished herbivore Coleoptera Cerambycidae Monochamus sp. 11.0 25.0 Siemann and Elser unpublished herbivore Coleoptera Cerambycidae Typocerus velutina 10.7 12.8 Siemann et al. 1996 herbivore Coleoptera Chrysomelidae Leptinotarsa decemlineata 10.1 12.0 Elser unpublished herbivore Coleoptera Chrysomelidae Paropsis atomaria 6.7 9.5 Fox and Macauley 1977 herbivore Coleoptera Curculionidae Pseudorhyncus sp. 9.3 2.7 Siemann et al. 1996 herbivore Coleoptera Curculionidae Sitona hispidula 10.4 4.0 Siemann et al. 1996 herbivore Coleoptera Elateridae genus sp. 12.3 17.5 Siemann and Elser unpublished herbivore Coleoptera Mordellidae Mordellistena sp. 9.7 2.1 Siemann et al. 1996 herbivore Coleoptera Scarabaeidae Hoplia modesta 12.3 6.8 Siemann et al. 1996 herbivore Coleoptera Scarabaeidae Phyllophaga sp. 10.4 20.0 Siemann and Elser unpublished herbivore Coleoptera Tenebrionidae Tenebrio molitor 8.0 14.5 Barker et al. 1998 herbivore Panorpida Bibionidae Bibio sp. 10.8 5.7 Siemann et al. 1996 herbivore Panorpida Chloropidae Oscinella sp. 9.3 2.2 Siemann et al. 1996 herbivore Panorpida Chloropidae Oscinella pube 9.7 4.2 Siemann et al. 1996 herbivore Panorpida Drosophilidae Drosophila arizonae 8.8 4.1 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila hydei 7.7 3.5 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila mojavensis 6.7 3.3 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila nigrospiracula 7.9 3.5 Markow et al. -

Hastings Slide Collection3
HASTINGS NATURAL HISTORY RESERVATION SLIDE COLLECTION 1 ORDER FAMILY GENUS SPECIES SUBSPECIES AUTHOR DATE # SLIDES COMMENTS/CORRECTIONS Siphonaptera Ceratophyllidae Diamanus montanus Baker 1895 221 currently Oropsylla (Diamanus) montana Siphonaptera Ceratophyllidae Diamanus spp. 1 currently Oropsylla (Diamanus) spp. Siphonaptera Ceratophyllidae Foxella ignota acuta Stewart 1940 402 syn. of F. ignota franciscana (Roths.) Siphonaptera Ceratophyllidae Foxella ignota (Baker) 1895 2 Siphonaptera Ceratophyllidae Foxella spp. 15 Siphonaptera Ceratophyllidae Malaraeus spp. 1 Siphonaptera Ceratophyllidae Malaraeus telchinum Rothschild 1905 491 M. telchinus Siphonaptera Ceratophyllidae Monopsyllus fornacis Jordan 1937 57 currently Eumolpianus fornacis Siphonaptera Ceratophyllidae Monopsyllus wagneri (Baker) 1904 131 currently Aetheca wagneri Siphonaptera Ceratophyllidae Monopsyllus wagneri ophidius Jordan 1929 2 syn. of Aetheca wagneri Siphonaptera Ceratophyllidae Opisodasys nesiotus Augustson 1941 2 Siphonaptera Ceratophyllidae Orchopeas sexdentatus (Baker) 1904 134 Siphonaptera Ceratophyllidae Orchopeas sexdentatus nevadensis (Jordan) 1929 15 syn. of Orchopeas agilis (Baker) Siphonaptera Ceratophyllidae Orchopeas spp. 8 Siphonaptera Ceratophyllidae Orchopeas latens (Jordan) 1925 2 Siphonaptera Ceratophyllidae Orchopeas leucopus (Baker) 1904 2 Siphonaptera Ctenophthalmidae Anomiopsyllus falsicalifornicus C. Fox 1919 3 Siphonaptera Ctenophthalmidae Anomiopsyllus congruens Stewart 1940 96 incl. 38 Paratypes; syn. of A. falsicalifornicus Siphonaptera -

The Evolution of Flea-Borne Transmission in Yersinia Pestis
Curr. Issues Mol. Biol. 7: 197–212. Online journal at www.cimb.org The Evolution of Flea-borne Transmission in Yersinia pestis B. Joseph Hinnebusch al., 1999; Hinchcliffe et al., 2003; Chain et al., 2004). Presumably, the change from the food- and water-borne Laboratory of Human Bacterial Pathogenesis, Rocky transmission of the Y. pseudotuberculosis ancestor to Mountain Laboratories, National Institute of Allergy the flea-borne transmission of Y. pestis occurred during and Infectious Diseases, National Institutes of Health, this evolutionarily short period of time. The monophyletic Hamilton, MT 59840 USA relationship of these two sister-species implies that the genetic changes that underlie the ability of Y. pestis to use Abstract the flea for its transmission vector are relatively few and Transmission by fleabite is a recent evolutionary adaptation discrete. Therefore, the Y. pseudotuberculosis –Y. pestis that distinguishes Yersinia pestis, the agent of plague, species complex provides an interesting case study in from Yersinia pseudotuberculosis and all other enteric the evolution of arthropod-borne transmission. Some of bacteria. The very close genetic relationship between Y. the genetic changes that led to flea-borne transmission pestis and Y. pseudotuberculosis indicates that just a few have been identified using the rat flea Xenopsylla cheopis discrete genetic changes were sufficient to give rise to flea- as model organism, and an evolutionary pathway can borne transmission. Y. pestis exhibits a distinct infection now be surmised. Reliance on the flea for transmission phenotype in its flea vector, and a transmissible infection also imposed new selective pressures on Y. pestis that depends on genes that are specifically required in the help explain the evolution of increased virulence in this flea, but not the mammal. -

ARTHROPODA Subphylum Hexapoda Protura, Springtails, Diplura, and Insects
NINE Phylum ARTHROPODA SUBPHYLUM HEXAPODA Protura, springtails, Diplura, and insects ROD P. MACFARLANE, PETER A. MADDISON, IAN G. ANDREW, JOCELYN A. BERRY, PETER M. JOHNS, ROBERT J. B. HOARE, MARIE-CLAUDE LARIVIÈRE, PENELOPE GREENSLADE, ROSA C. HENDERSON, COURTenaY N. SMITHERS, RicarDO L. PALMA, JOHN B. WARD, ROBERT L. C. PILGRIM, DaVID R. TOWNS, IAN McLELLAN, DAVID A. J. TEULON, TERRY R. HITCHINGS, VICTOR F. EASTOP, NICHOLAS A. MARTIN, MURRAY J. FLETCHER, MARLON A. W. STUFKENS, PAMELA J. DALE, Daniel BURCKHARDT, THOMAS R. BUCKLEY, STEVEN A. TREWICK defining feature of the Hexapoda, as the name suggests, is six legs. Also, the body comprises a head, thorax, and abdomen. The number A of abdominal segments varies, however; there are only six in the Collembola (springtails), 9–12 in the Protura, and 10 in the Diplura, whereas in all other hexapods there are strictly 11. Insects are now regarded as comprising only those hexapods with 11 abdominal segments. Whereas crustaceans are the dominant group of arthropods in the sea, hexapods prevail on land, in numbers and biomass. Altogether, the Hexapoda constitutes the most diverse group of animals – the estimated number of described species worldwide is just over 900,000, with the beetles (order Coleoptera) comprising more than a third of these. Today, the Hexapoda is considered to contain four classes – the Insecta, and the Protura, Collembola, and Diplura. The latter three classes were formerly allied with the insect orders Archaeognatha (jumping bristletails) and Thysanura (silverfish) as the insect subclass Apterygota (‘wingless’). The Apterygota is now regarded as an artificial assemblage (Bitsch & Bitsch 2000).