American Museum Novitates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insect Orders V: Panorpida & Hymenoptera
Insect Orders V: Panorpida & Hymenoptera • The Panorpida contain 5 orders: the Mecoptera, Siphonaptera, Diptera, Trichoptera and Lepidoptera. • Available evidence clearly indicates that the Lepidoptera and the Trichoptera are sister groups. • The Siphonaptera and Mecoptera are also closely related but it is not clear whether the Siponaptera is the sister group of all of the Mecoptera or a group (Boreidae) within the Mecoptera. If the latter is true, then the Mecoptera is paraphyletic as currently defined. • The Diptera is the sister group of the Siphonaptera + Mecoptera and together make up the Mecopteroids. • The Hymenoptera does not appear to be closely related to any of the other holometabolous orders. Mecoptera (Scorpionflies, hangingflies) • Classification. 600 species worldwide, arranged into 9 families (5 in the US). A very old group, many fossils from the Permian (260 mya) onward. • Structure. Most distinctive feature is the elongated clypeus and labrum that together form a rostrum. The order gets its common name from the gential segment of the male in the family Panorpodiae, which is bulbous and often curved forward above the abdomen, like the sting of a scorpion. Larvae are caterpillar-like or grub- like. • Natural history. Scorpionflies are most common in cool, moist habitats. They get the name “hangingflies” from their habit of hanging upside down on vegetation. Larvae and adult males are mostly predators or scavengers. Adult females are usually scavengers. Larvae and adults in some groups may feed on vegetation. Larvae of most species are terrestrial and caterpillar-like in body form. Larvae of some species are aquatic. In the family Bittacidae males attract females for mating by releasing a sex pheromone and then presenting the female with a nuptial gift. -
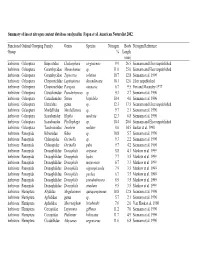
Summary of Insect Nitrogen Content Database Analyzed in Fagan Et Al
Summary of insect nitrogen content database analyzed in Fagan et al. American Naturalist 2002. Functional Ordinal Grouping Family Genus Species Nitrogen Body Nitrogen Reference Group % Length (mm) herbivore Coleoptera Buprestidae Chalcophora virginiensis 9.9 26.5 Siemann and Elser unpublished herbivore Coleoptera Cerambycidae Monochamus sp. 11.0 25.0 Siemann and Elser unpublished herbivore Coleoptera Cerambycidae Typocerus velutina 10.7 12.8 Siemann et al. 1996 herbivore Coleoptera Chrysomelidae Leptinotarsa decemlineata 10.1 12.0 Elser unpublished herbivore Coleoptera Chrysomelidae Paropsis atomaria 6.7 9.5 Fox and Macauley 1977 herbivore Coleoptera Curculionidae Pseudorhyncus sp. 9.3 2.7 Siemann et al. 1996 herbivore Coleoptera Curculionidae Sitona hispidula 10.4 4.0 Siemann et al. 1996 herbivore Coleoptera Elateridae genus sp. 12.3 17.5 Siemann and Elser unpublished herbivore Coleoptera Mordellidae Mordellistena sp. 9.7 2.1 Siemann et al. 1996 herbivore Coleoptera Scarabaeidae Hoplia modesta 12.3 6.8 Siemann et al. 1996 herbivore Coleoptera Scarabaeidae Phyllophaga sp. 10.4 20.0 Siemann and Elser unpublished herbivore Coleoptera Tenebrionidae Tenebrio molitor 8.0 14.5 Barker et al. 1998 herbivore Panorpida Bibionidae Bibio sp. 10.8 5.7 Siemann et al. 1996 herbivore Panorpida Chloropidae Oscinella sp. 9.3 2.2 Siemann et al. 1996 herbivore Panorpida Chloropidae Oscinella pube 9.7 4.2 Siemann et al. 1996 herbivore Panorpida Drosophilidae Drosophila arizonae 8.8 4.1 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila hydei 7.7 3.5 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila mojavensis 6.7 3.3 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila nigrospiracula 7.9 3.5 Markow et al. -

Fleas (Siphonaptera) Are Cretaceous, and Evolved with Theria
bioRxiv preprint doi: https://doi.org/10.1101/014308; this version posted January 24, 2015. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Fleas (Siphonaptera) are Cretaceous, and Evolved with Theria Qiyun Zhu1, Michael Hastriter2, Michael Whiting2, 3, Katharina Dittmar1, 4* Jan. 23, 2015 Abstract: Fleas (order Siphonaptera) are highly-specialized, diverse blood-feeding ectoparasites of mammals and birds with an enigmatic evolutionary history and obscure origin. We here present a molecular phylogenetic study based on a compre- hensive taxon sampling of 259 flea taxa, representing 16 of the 18 extant families of this order. A Bayesian phylogenetic tree with strong nodal support was recovered, consisting of seven sequentially derived lineages with Macropsyllidae at the base and Stephanocircidae as the second basal group. Divergence times of flea lineages were estimated based on fossil records and host specific associations to bats (Chiroptera), showing that the common ancestor of extant Siphonaptera split from its clos- est mecopteran sister group in the Early Cretaceous and basal lineages diversified during the Late Cretaceous. However, most of the intraordinal divergence into families took place after the K-Pg boundary. Ancestral states of host association and bioge- ographical distribution were reconstructed, suggesting with high likelihood that fleas originated in the southern continents (Gondwana) and migrated from South America to their extant distributions in a relatively short time frame. Theria (placental mammals and marsupials) represent the most likely ancestral host group of extant Siphonaptera, with marsupials occupying a more important role than previously assumed. -

Mecoptera: Meropeidae): Simply Dull Or Just Inscrutable?
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Center for Systematic Entomology, Gainesville, Insecta Mundi Florida 8-24-2007 Etymology of the earwigfly, Merope tuber Newman (Mecoptera: Meropeidae): Simply dull or just inscrutable? Louis A. Somma University of Florida, [email protected] James C. Dunford University of Florida, Gainesville, FL Follow this and additional works at: https://digitalcommons.unl.edu/insectamundi Part of the Entomology Commons Somma, Louis A. and Dunford, James C., "Etymology of the earwigfly, Merope tuber Newman (Mecoptera: Meropeidae): Simply dull or just inscrutable?" (2007). Insecta Mundi. 65. https://digitalcommons.unl.edu/insectamundi/65 This Article is brought to you for free and open access by the Center for Systematic Entomology, Gainesville, Florida at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Insecta Mundi by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. INSECTA MUNDI A Journal of World Insect Systematics 0013 Etymology of the earwigfly, Merope tuber Newman (Mecoptera: Meropeidae): Simply dull or just inscrutable? Louis A. Somma Department of Zoology PO Box 118525 University of Florida Gainesville, FL 32611-8525 [email protected] James C. Dunford Department of Entomology and Nematology PO Box 110620, IFAS University of Florida Gainesville, FL 32611-0620 [email protected] Date of Issue: August 24, 2007 CENTER FOR SYSTEMATIC ENTOMOLOGY, INC., Gainesville, FL Louis A. Somma and James C. Dunford Etymology of the earwigfly, Merope tuber Newman (Mecoptera: Meropeidae): Simply dull or just inscrutable? Insecta Mundi 0013: 1-5 Published in 2007 by Center for Systematic Entomology, Inc. P. O. Box 147100 Gainesville, FL 32604-7100 U. -

Allometric and Phylogenetic Variation in Insect Phosphorus 18, 103–109 Content
Functional Blackwell Publishing, Ltd. Ecology 2004 Allometric and phylogenetic variation in insect phosphorus 18, 103–109 content H. A. WOODS,*† W. F. FAGAN,‡ J. J. ELSER§ and J. F. HARRISON§ *Section of Integrative Biology C0930, University of Texas at Austin, Austin, TX 78712, USA, ‡Department of Biology, University of Maryland, College Park, MD 20742, USA, and §Department of Biology, Arizona State University, Tempe, AZ 85287–1501, USA Summary 1. Phosphorus content was measured in adult insects and arachnids from 170 species collected in the Sonoran Desert. 2. Across insect body sizes spanning four orders of magnitude, phosphorus content was inversely related to body mass. The largest species (∼1 g dry) had phosphorus contents that were only about 60% (0·62% P absolute) as high as phosphorus contents of the smallest species (∼0·0001 g dry; 0·97% P). Negative phosphorus allometry was observed within each of seven insect orders and within arachnids. 3. Phosphorus contents of insect predators and herbivores were statistically indistinguishable. 4. More recently derived orders tended to have lower phosphorus contents – with the exception of the most recently derived group (Panorpida = Diptera + Lepidoptera), which had high phosphorus contents. Key-words: Allometry, body size, exoskeleton, phosphorus limitation, stoichiometry Functional Ecology (2004) 18, 103–109 processes dependent on elemental composition (e.g. Introduction nutrient cycling) and because P occupies a key position Herbivorous insects face a fundamental asymmetry in the recently developed theory of ecological stoichio- between the nutrient contents of their body tissues and metry (Sterner & Elser 2002). those of the plants they eat (Slansky & Feeny 1977; Several expectations can be derived a priori by McNeill & Southwood 1978; Mattson 1980; Strong, extensions of previous work on N. -

Phylogeny of Endopterygote Insects, the Most Successful Lineage of Living Organisms*
REVIEW Eur. J. Entomol. 96: 237-253, 1999 ISSN 1210-5759 Phylogeny of endopterygote insects, the most successful lineage of living organisms* N iels P. KRISTENSEN Zoological Museum, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen 0, Denmark; e-mail: [email protected] Key words. Insecta, Endopterygota, Holometabola, phylogeny, diversification modes, Megaloptera, Raphidioptera, Neuroptera, Coleóptera, Strepsiptera, Díptera, Mecoptera, Siphonaptera, Trichoptera, Lepidoptera, Hymenoptera Abstract. The monophyly of the Endopterygota is supported primarily by the specialized larva without external wing buds and with degradable eyes, as well as by the quiescence of the last immature (pupal) stage; a specialized morphology of the latter is not an en dopterygote groundplan trait. There is weak support for the basal endopterygote splitting event being between a Neuropterida + Co leóptera clade and a Mecopterida + Hymenoptera clade; a fully sclerotized sitophore plate in the adult is a newly recognized possible groundplan autapomorphy of the latter. The molecular evidence for a Strepsiptera + Díptera clade is differently interpreted by advo cates of parsimony and maximum likelihood analyses of sequence data, and the morphological evidence for the monophyly of this clade is ambiguous. The basal diversification patterns within the principal endopterygote clades (“orders”) are succinctly reviewed. The truly species-rich clades are almost consistently quite subordinate. The identification of “key innovations” promoting evolution -

2015-2025 Pennsylvania Wildlife Action Plan
2 0 1 5 – 2 0 2 5 Species Assessments Appendix 1.1A – Birds A Comprehensive Status Assessment of Pennsylvania’s Avifauna for Application to the State Wildlife Action Plan Update 2015 (Jason Hill, PhD) Assessment of eBird data for the importance of Pennsylvania as a bird migratory corridor (Andy Wilson, PhD) Appendix 1.1B – Mammals A Comprehensive Status Assessment of Pennsylvania’s Mammals, Utilizing NatureServe Ranking Methodology and Rank Calculator Version 3.1 for Application to the State Wildlife Action Plan Update 2015 (Charlie Eichelberger and Joe Wisgo) Appendix 1.1C – Reptiles and Amphibians A Revision of the State Conservation Ranks of Pennsylvania’s Herpetofauna Appendix 1.1D – Fishes A Revision of the State Conservation Ranks of Pennsylvania’s Fishes Appendix 1.1E – Invertebrates Invertebrate Assessment for the 2015 Pennsylvania Wildlife Action Plan Revision 2015-2025 Pennsylvania Wildlife Action Plan Appendix 1.1A - Birds A Comprehensive Status Assessment of Pennsylvania’s Avifauna for Application to the State Wildlife Action Plan Update 2015 Jason M. Hill, PhD. Table of Contents Assessment ............................................................................................................................................. 3 Data Sources ....................................................................................................................................... 3 Species Selection ................................................................................................................................ -

Hemoglobin-Derived Porphyrins Preserved in a Middle Eocene Blood-Engorged Mosquito
Hemoglobin-derived porphyrins preserved in a Middle Eocene blood-engorged mosquito Dale E. Greenwalta,1, Yulia S. Gorevab, Sandra M. Siljeströmb,c,d, Tim Roseb, and Ralph E. Harbache Departments of aPaleobiology and bMineral Sciences, National Museum of Natural History, Washington, DC 20013; cGeophysical Laboratory, Carnegie Institution of Washington, Washington, DC 20015; dDepartment of Chemistry, Materials, and Surfaces, SP Technical Research Institute of Sweden, 501 11 Borås, Sweden; and eDepartment of Life Sciences, Natural History Museum, London SW7 5BD, United Kingdom Edited by Michael S. Engel, University of Kansas, Lawrence, KS, and accepted by the Editorial Board September 18, 2013 (received for review June 7, 2013) Although hematophagy is found in ∼14,000 species of extant vertebrate hosts (15). Given the similarities of the fossilized insects, the fossil record of blood-feeding insects is extremely poor trypanosomes to known extant heteroxenous species, and the and largely confined to specimens identified as hematophagic hematophagic lifestyle of the extant relatives of the insect hosts, based on their taxonomic affinities with extant hematophagic Poinar has concluded that these fossils represent examples of insects; direct evidence of hematophagy is limited to four insect hematophagy. Even more direct evidence of hematophagy is the fossils in which trypanosomes and the malarial protozoan Plasmo- observation of nucleated erythrocytes containing putative para- dium have been found. Here, we describe a blood-engorged mos- sitophorous vacuoles in the gut of an amber-embedded sandfly(16). quito from the Middle Eocene Kishenehn Formation in Montana. Poinar has also reported the presence of Plasmodium spor- This unique specimen provided the opportunity to ask whether or ozoites in the salivary gland and salivary gland ducts of a fossil not hemoglobin, or biomolecules derived from hemoglobin, were female mosquito of the genus Culex, some extant species of preserved in the fossilized blood meal. -

Changes to the Fossil Record of Insects Through Fifteen Years of Discovery
This is a repository copy of Changes to the Fossil Record of Insects through Fifteen Years of Discovery. White Rose Research Online URL for this paper: https://eprints.whiterose.ac.uk/88391/ Version: Published Version Article: Nicholson, David Blair, Mayhew, Peter John orcid.org/0000-0002-7346-6560 and Ross, Andrew J (2015) Changes to the Fossil Record of Insects through Fifteen Years of Discovery. PLosOne. e0128554. https://doi.org/10.1371/journal.pone.0128554 Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ RESEARCH ARTICLE Changes to the Fossil Record of Insects through Fifteen Years of Discovery David B. Nicholson1,2¤*, Peter J. Mayhew1, Andrew J. Ross2 1 Department of Biology, University of York, York, United Kingdom, 2 Department of Natural Sciences, National Museum of Scotland, Edinburgh, United Kingdom ¤ Current address: Department of Earth Sciences, The Natural History Museum, London, United Kingdom * [email protected] Abstract The first and last occurrences of hexapod families in the fossil record are compiled from publications up to end-2009. -

New Species of Neopanorpa (Mecoptera) from Vietnam, with a Key to the Species of Mecoptera of Vietnam Author(S): Wesley J
New Species of Neopanorpa (Mecoptera) from Vietnam, with a Key to the Species of Mecoptera of Vietnam Author(s): Wesley J. Bicha, Nathan Schiff, Thai Hong Pham, Aaron Lancaster and Brian Scheffler Source: Proceedings of the Entomological Society of Washington, 119(4):529-544. Published By: Entomological Society of Washington https://doi.org/10.4289/0013-8797.119.4.529 URL: http://www.bioone.org/doi/full/10.4289/0013-8797.119.4.529 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/ terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. PROC. ENTOMOL. SOC. WASH. 119(4), 2017, pp. 529–544 NEW SPECIES OF NEOPANORPA (MECOPTERA) FROM VIETNAM, WITH A KEY TO THE SPECIES OF MECOPTERA OF VIETNAM urn:lsid:zoobank.org:pub:CE24D561-5F0E-482F-953D-B0B809D4A607 WESLEY J. BICHA,NATHAN SCHIFF,THAI HONG PHAM,AARON LANCASTER, AND BRIAN SCHEFFLER (WJB) Tropical Research Associates Entomology, 521 46th Street, Sacramento, California 95819 (e-mail: [email protected]); (NS) United States Department of Agriculture Forest Service, Southern Research Station, Center for Bottomland Hardwoods Research, P.O. -

Madygen, Triassic Lagerstätte Number One, Before and After Sharov
ALAVESIA, 2: 113-124 (2008) ISSN 1887-7419 Madygen, Triassic Lagerstätte number one, before and after Sharov Dmitry E. SHCHERBAKOV Paleontological Institute, Russian Academy of Sciences, Profsoyuznaya 123, Moscow 117647, Russia. E-mail: [email protected] ABSTRACT The insect fauna of the world’s richest Triassic fossil locality, Madygen (Ladinian–Carnian of Kyrgyzstan) is reviewed; other groups of animals and plants recorded from the locality are also listed. The research history, fossil preservation and paleoenvironment of the Madygen Formation are briefly discussed. The site was discovered in 1933, and the better part of fossils was collected from the outcrop richest in insects, Dzhayloucho, during five expeditions headed by Alexander Sharov, who discovered there and described two peculiar gliding reptiles that made Madygen worldwide known. The entomofauna includes 20 orders (including the earliest Hymenoptera and early Diptera) and nearly 100 families. The insect assemblage is numerically dominated by Coleoptera, Blattodea, and Auchenorrhyncha. In Dzhayloucho, subdo- minants are Mecoptera, Orthoptera, and Protorthoptera. The largest insects belong to Titanoptera, the order established by Sharov and the most diverse in Madygen. Amphibiotic insects are rare and represented almost exclusively by adults. In some outcrops phyllopod Kazacharthra are common. The paleoenvironment may be reconstructed as an intermontane river valley in seasonally arid climate, with mineralized oxbow lakes and ephemeral ponds on the floodplain. KEY WORDS: Middle–Late Triassic. Insects. Composition of entomofauna. Paleoenvironment. INTRODUCTION 1966; cited after Dobruskina 1995). According to her ideas The world renown fossil site near the village of Mady- the Madygen Formation contains both Permian and Trias- gen, in foothills of the Turkestan Range (south of Fergana sic strata (cropping out in different areas), and the Permian Valley), Kyrgyzstan has yielded more than twenty thousand Madygen flora was rich in Mesozoic elements. -

Use of Fine-Scale Stratigraphy and Chemostratigraphy to Evaluate
J Paleolimnol (2010) 44:645–666 DOI 10.1007/s10933-010-9445-1 ORIGINALPAPER Use of fine-scale stratigraphy and chemostratigraphy to evaluate conditions of deposition and preservation of a Triassic Lagersta¨tte, south-central Virginia C. M. Liutkus • J. S. Beard • N. C. Fraser • P. C. Ragland Received: 13 March 2009 / Accepted: 19 May 2010 / Published online: 5 June 2010 Ó Springer Science+Business Media B.V. 2010 Abstract The rich, fossiliferous Triassic sediments surrounding lithologies reveal a change from silici- exposed in the Virginia Solite Quarry include a 34- clastic-dominated layers (Unit 1) to dolomite-silici- mm-thick ‘‘insect layer’’ that is notable for detailed clastic laminites above (Unit 2 and the insect layer), preservation of soft-bodied invertebrate and verte- separated by a boundary dolostone layer that is brate remains. We describe this unique Konservat- traceable for over 200 m. We interpret this sedimen- Lagersta¨tte and use sedimentologic and geochemical tary shift as the initial stages in the transgression of a analyses to interpret the environmental conditions shallow, saline, alkaline rift-basin lake over lake necessary to preserve such delicate fossils. This work margin deposits. The absence of bioturbation by is among the first attempts to apply detailed plants and benthic organisms, as well as a lack of geochemical/stratigraphic analysis to the study of predation on the insects, is not explained by signif- Lagersta¨tten and we report on a 332-mm-thick icant water depth, but is instead more reasonably section that includes the insect layer and the rocks considered a result of the chemistry of the water at immediately below and above it.