A Chromosome-Level Assembly of the Cat Flea Genome Uncovers Rampant Gene
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insect Orders V: Panorpida & Hymenoptera
Insect Orders V: Panorpida & Hymenoptera • The Panorpida contain 5 orders: the Mecoptera, Siphonaptera, Diptera, Trichoptera and Lepidoptera. • Available evidence clearly indicates that the Lepidoptera and the Trichoptera are sister groups. • The Siphonaptera and Mecoptera are also closely related but it is not clear whether the Siponaptera is the sister group of all of the Mecoptera or a group (Boreidae) within the Mecoptera. If the latter is true, then the Mecoptera is paraphyletic as currently defined. • The Diptera is the sister group of the Siphonaptera + Mecoptera and together make up the Mecopteroids. • The Hymenoptera does not appear to be closely related to any of the other holometabolous orders. Mecoptera (Scorpionflies, hangingflies) • Classification. 600 species worldwide, arranged into 9 families (5 in the US). A very old group, many fossils from the Permian (260 mya) onward. • Structure. Most distinctive feature is the elongated clypeus and labrum that together form a rostrum. The order gets its common name from the gential segment of the male in the family Panorpodiae, which is bulbous and often curved forward above the abdomen, like the sting of a scorpion. Larvae are caterpillar-like or grub- like. • Natural history. Scorpionflies are most common in cool, moist habitats. They get the name “hangingflies” from their habit of hanging upside down on vegetation. Larvae and adult males are mostly predators or scavengers. Adult females are usually scavengers. Larvae and adults in some groups may feed on vegetation. Larvae of most species are terrestrial and caterpillar-like in body form. Larvae of some species are aquatic. In the family Bittacidae males attract females for mating by releasing a sex pheromone and then presenting the female with a nuptial gift. -

Fleas, Hosts and Habitat: What Can We Predict About the Spread of Vector-Borne Zoonotic Diseases?
2010 Fleas, Hosts and Habitat: What can we predict about the spread of vector-borne zoonotic diseases? Ph.D. Dissertation Megan M. Friggens School of Forestry I I I \, l " FLEAS, HOSTS AND HABITAT: WHAT CAN WE PREDICT ABOUT THE SPREAD OF VECTOR-BORNE ZOONOTIC DISEASES? by Megan M. Friggens A Dissertation Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Forest Science Northern Arizona University May 2010 ?Jii@~-~-u-_- Robert R. Parmenter, Ph. D. ~",l(*~ l.~ Paulette L. Ford, Ph. D. --=z:r-J'l1jU~ David M. Wagner, Ph. D. ABSTRACT FLEAS, HOSTS AND HABITAT: WHAT CAN WE PREDICT ABOUT THE SPREAD OF VECTOR-BORNE ZOONOTIC DISEASES? MEGAN M. FRIGGENS Vector-borne diseases of humans and wildlife are experiencing resurgence across the globe. I examine the dynamics of flea borne diseases through a comparative analysis of flea literature and analyses of field data collected from three sites in New Mexico: The Sevilleta National Wildlife Refuge, the Sandia Mountains and the Valles Caldera National Preserve (VCNP). My objectives were to use these analyses to better predict and manage for the spread of diseases such as plague (Yersinia pestis). To assess the impact of anthropogenic disturbance on flea communities, I compiled and analyzed data from 63 published empirical studies. Anthropogenic disturbance is associated with conditions conducive to increased transmission of flea-borne diseases. Most measures of flea infestation increased with increasing disturbance or peaked at intermediate levels of disturbance. Future trends of habitat and climate change will probably favor the spread of flea-borne disease. -

Flower Preferences and Pollen Transport Networks for Cavity‐Nesting Solitary Bees: Implications for the Design of Agri‐Envir
Received: 14 February 2018 | Revised: 22 April 2018 | Accepted: 23 April 2018 DOI: 10.1002/ece3.4234 ORIGINAL RESEARCH Flower preferences and pollen transport networks for cavity- nesting solitary bees: Implications for the design of agri- environment schemes Catherine E. A. Gresty1 | Elizabeth Clare2 | Dion S. Devey3 | Robyn S. Cowan3 | Laszlo Csiba3 | Panagiota Malakasi3 | Owen T. Lewis1 | Katherine J. Willis1,3 1Department of Zoology, University of Oxford, Oxford, UK Abstract 2School of Biological and Chemical Floral foraging resources are valuable for pollinator conservation on farmland, and Sciences, Queen Mary University of London, their provision is encouraged by agri- environment schemes in many countries. Across London, UK Europe, wildflower seed mixtures are widely sown on farmland to encourage pollina- 3Royal Botanic Gardens, Kew, Richmond, UK tors, but the extent to which key pollinator groups such as solitary bees exploit and Correspondence benefit from these resources is unclear. We used high- throughput sequencing of 164 Catherine E. A. Gresty, Department of Zoology, New Radcliffe House, Radcliffe pollen samples extracted from the brood cells of six common cavity- nesting solitary Observatory Quarter, 6GG, Woodstock Rd, bee species (Osmia bicornis, Osmia caerulescens, Megachile versicolor, Megachile Oxford OX2, UK. Email: [email protected] ligniseca, Megachile centuncularis and Hylaeus confusus) which are widely distributed across the UK and Europe. We documented their pollen use across 19 farms in south- ern England, UK, revealing their forage plants and examining the structure of their pollen transport networks. Of the 32 plant species included currently in sown wild- flower mixes, 15 were recorded as present within close foraging range of the bees on the study farms, but only Ranunculus acris L. -

Fleas and Flea-Borne Diseases
International Journal of Infectious Diseases 14 (2010) e667–e676 Contents lists available at ScienceDirect International Journal of Infectious Diseases journal homepage: www.elsevier.com/locate/ijid Review Fleas and flea-borne diseases Idir Bitam a, Katharina Dittmar b, Philippe Parola a, Michael F. Whiting c, Didier Raoult a,* a Unite´ de Recherche en Maladies Infectieuses Tropicales Emergentes, CNRS-IRD UMR 6236, Faculte´ de Me´decine, Universite´ de la Me´diterrane´e, 27 Bd Jean Moulin, 13385 Marseille Cedex 5, France b Department of Biological Sciences, SUNY at Buffalo, Buffalo, NY, USA c Department of Biology, Brigham Young University, Provo, Utah, USA ARTICLE INFO SUMMARY Article history: Flea-borne infections are emerging or re-emerging throughout the world, and their incidence is on the Received 3 February 2009 rise. Furthermore, their distribution and that of their vectors is shifting and expanding. This publication Received in revised form 2 June 2009 reviews general flea biology and the distribution of the flea-borne diseases of public health importance Accepted 4 November 2009 throughout the world, their principal flea vectors, and the extent of their public health burden. Such an Corresponding Editor: William Cameron, overall review is necessary to understand the importance of this group of infections and the resources Ottawa, Canada that must be allocated to their control by public health authorities to ensure their timely diagnosis and treatment. Keywords: ß 2010 International Society for Infectious Diseases. Published by Elsevier Ltd. All rights reserved. Flea Siphonaptera Plague Yersinia pestis Rickettsia Bartonella Introduction to 16 families and 238 genera have been described, but only a minority is synanthropic, that is they live in close association with The past decades have seen a dramatic change in the geographic humans (Table 1).4,5 and host ranges of many vector-borne pathogens, and their diseases. -

Arthropod Parasites in Domestic Animals
ARTHROPOD PARASITES IN DOMESTIC ANIMALS Abbreviations KINGDOM PHYLUM CLASS ORDER CODE Metazoa Arthropoda Insecta Siphonaptera INS:Sip Mallophaga INS:Mal Anoplura INS:Ano Diptera INS:Dip Arachnida Ixodida ARA:Ixo Mesostigmata ARA:Mes Prostigmata ARA:Pro Astigmata ARA:Ast Crustacea Pentastomata CRU:Pen References Ashford, R.W. & Crewe, W. 2003. The parasites of Homo sapiens: an annotated checklist of the protozoa, helminths and arthropods for which we are home. Taylor & Francis. Taylor, M.A., Coop, R.L. & Wall, R.L. 2007. Veterinary Parasitology. 3rd edition, Blackwell Pub. HOST-PARASITE CHECKLIST Class: MAMMALIA [mammals] Subclass: EUTHERIA [placental mammals] Order: PRIMATES [prosimians and simians] Suborder: SIMIAE [monkeys, apes, man] Family: HOMINIDAE [man] Homo sapiens Linnaeus, 1758 [man] ARA:Ast Sarcoptes bovis, ectoparasite (‘milker’s itch’)(mange mite) ARA:Ast Sarcoptes equi, ectoparasite (‘cavalryman’s itch’)(mange mite) ARA:Ast Sarcoptes scabiei, skin (mange mite) ARA:Ixo Ixodes cornuatus, ectoparasite (scrub tick) ARA:Ixo Ixodes holocyclus, ectoparasite (scrub tick, paralysis tick) ARA:Ixo Ornithodoros gurneyi, ectoparasite (kangaroo tick) ARA:Pro Cheyletiella blakei, ectoparasite (mite) ARA:Pro Cheyletiella parasitivorax, ectoparasite (rabbit fur mite) ARA:Pro Demodex brevis, sebacceous glands (mange mite) ARA:Pro Demodex folliculorum, hair follicles (mange mite) ARA:Pro Trombicula sarcina, ectoparasite (black soil itch mite) INS:Ano Pediculus capitis, ectoparasite (head louse) INS:Ano Pediculus humanus, ectoparasite (body -

Flea NEWS 56 Department of Entomology Iowa State University, Ames, Iowa 50011 50, June, 1995; No
flea NEWS 56 Department of Entomology Iowa State University, Ames, Iowa 50011 50, June, 1995; No. 51, December, 1995; No. 52, June, 1996, No. 53, December, Table of Contents 1996; No. 54, June, 1997, 55, January, 1998 and this number. Literature..............................662 Mailing List Changes .............668 ❊❄❊❄❊❄❊ Miscellanea...........................660 MISCELLANEA Flea News (Online) has now been FLEA NEWS is a biannual newsletter assigned the following International devoted to matters involving insects Standard Serial Number: ISSN 1089- belonging to the order Siphonaptera (fleas) 7631 and related subjects. It is compiled and distributed free of charge by Robert E. Lewis ❖❏❖❏❖❏❖ <[email protected]> in cooperation with the Department of Entomology at Iowa State Dr. Glen Chilton of the Department University, Ames, IA, and a grant in aid of Biology, St. Mary's College, Calg- from Wellmark International. ary, Alberta, T2S 2N5, Canada, rec- Flea News is mainly bibliographic in nature. Many of the sources are abstracting ently called my attention to the Birds journals and title pages and not all citations of North America accounts published have been checked for completeness or jointly by the American Ornithol- accuracy. Additional information will be ogists' Union and the Academy of provided upon written or e-mail request. Natural Sciences, Philadelphia. To Further, recipients are urged to contribute date 320 accounts have been publish- items of interest to the professon for ed and the following titles include inclusion herein. information on fleas: This newsletter is now available in 7. Northern Mockingbird electronic format. The preferred method of 11. Tree Swallow accessing the electronic version is through the 12. -

Population Variability of Rotylenchulus Reniformis in Cotton Agroecosystems Megan Leach Clemson University, [email protected]
Clemson University TigerPrints All Dissertations Dissertations 12-2010 Population Variability of Rotylenchulus reniformis in Cotton Agroecosystems Megan Leach Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_dissertations Part of the Plant Pathology Commons Recommended Citation Leach, Megan, "Population Variability of Rotylenchulus reniformis in Cotton Agroecosystems" (2010). All Dissertations. 669. https://tigerprints.clemson.edu/all_dissertations/669 This Dissertation is brought to you for free and open access by the Dissertations at TigerPrints. It has been accepted for inclusion in All Dissertations by an authorized administrator of TigerPrints. For more information, please contact [email protected]. POPULATION VARIABILITY OF ROTYLENCHULUS RENIFORMIS IN COTTON AGROECOSYSTEMS A Dissertation Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Plant and Environmental Sciences by Megan Marie Leach December 2010 Accepted by: Dr. Paula Agudelo, Committee Chair Dr. Halina Knap Dr. John Mueller Dr. Amy Lawton-Rauh Dr. Emerson Shipe i ABSTRACT Rotylenchulus reniformis, reniform nematode, is a highly variable species and an economically important pest in many cotton fields across the southeast. Rotation to resistant or poor host crops is a prescribed method for management of reniform nematode. An increase in the incidence and prevalence of the nematode in the United States has been reported over the -

DOMESTIC RATS, FLEAS and NATIVE RODENTS
DOMESTIC RATS, FLEAS and NATIVE RODENTS In Relation To Plague In The United States By Entomologist Carl 0. Mohr INTRODUCTION and finally to the lungs causing pneumonic plague. ubonic plague is a rodent and rodent - Pneumonic plague is extremely fatal and flea disease caused by the plague bacil highly infectious when sputum droplets pass Blus Pasturella pest is which is transmitted direct from person to person. The death from animal to animal and thence to man by rate due to it is practically 100 percent. fleas. It is highly fatal. At least half Plague is dreaded particularly where of the human cases result in death without living conditions are such as to bring modern medication. (Table I — last two human beings into close contact with large columns). Because of their close associa* oriental-rat-flea populations, and where tion with man, domestic rats* and their crowded conditions permit rapid pneumonic fleas, especially the oriental rat flea transmission from man to man. Xenopsylla cheopis, are responsible for most human epidemics. Only occasional cases ANCIENT AMERICAN DISEASE OR RECENT are caused by bites of other fleas or by INTRODUCTION direct infection from handling rodents. Infection due to bites of fleas or due to Two widely different views exist con direct contact commonly results in swollen cerning the arrival of plague in North lymph glands, called buboes, hence the name America. The prevalent view is that it was bubonic plague. Infection may progress to introduced from the Orient into North the blood stream causing septicemic plague, America at San Francisco through ship- * Rattus rattus and Rattus norvegicus. -

Smithsonian Miscellaneous Collections
SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 104, NUMBER 7 THE FEEDING APPARATUS OF BITING AND SUCKING INSECTS AFFECTING MAN AND ANIMALS BY R. E. SNODGRASS Bureau of Entomology and Plant Quarantine Agricultural Research Administration U. S. Department of Agriculture (Publication 3773) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION OCTOBER 24, 1944 SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 104, NUMBER 7 THE FEEDING APPARATUS OF BITING AND SUCKING INSECTS AFFECTING MAN AND ANIMALS BY R. E. SNODGRASS Bureau of Entomology and Plant Quarantine Agricultural Research Administration U. S. Department of Agriculture P£R\ (Publication 3773) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION OCTOBER 24, 1944 BALTIMORE, MO., U. S. A. THE FEEDING APPARATUS OF BITING AND SUCKING INSECTS AFFECTING MAN AND ANIMALS By R. E. SNODGRASS Bureau of Entomology and Plant Quarantine, Agricultural Research Administration, U. S. Department of Agriculture CONTENTS Page Introduction 2 I. The cockroach. Order Orthoptera 3 The head and the organs of ingestion 4 General structure of the head and mouth parts 4 The labrum 7 The mandibles 8 The maxillae 10 The labium 13 The hypopharynx 14 The preoral food cavity 17 The mechanism of ingestion 18 The alimentary canal 19 II. The biting lice and booklice. Orders Mallophaga and Corrodentia. 21 III. The elephant louse 30 IV. The sucking lice. Order Anoplura 31 V. The flies. Order Diptera 36 Mosquitoes. Family Culicidae 37 Sand flies. Family Psychodidae 50 Biting midges. Family Heleidae 54 Black flies. Family Simuliidae 56 Net-winged midges. Family Blepharoceratidae 61 Horse flies. Family Tabanidae 61 Snipe flies. Family Rhagionidae 64 Robber flies. Family Asilidae 66 Special features of the Cyclorrhapha 68 Eye gnats. -
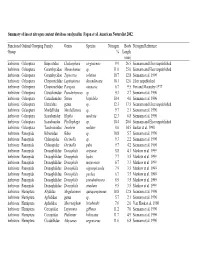
Summary of Insect Nitrogen Content Database Analyzed in Fagan Et Al
Summary of insect nitrogen content database analyzed in Fagan et al. American Naturalist 2002. Functional Ordinal Grouping Family Genus Species Nitrogen Body Nitrogen Reference Group % Length (mm) herbivore Coleoptera Buprestidae Chalcophora virginiensis 9.9 26.5 Siemann and Elser unpublished herbivore Coleoptera Cerambycidae Monochamus sp. 11.0 25.0 Siemann and Elser unpublished herbivore Coleoptera Cerambycidae Typocerus velutina 10.7 12.8 Siemann et al. 1996 herbivore Coleoptera Chrysomelidae Leptinotarsa decemlineata 10.1 12.0 Elser unpublished herbivore Coleoptera Chrysomelidae Paropsis atomaria 6.7 9.5 Fox and Macauley 1977 herbivore Coleoptera Curculionidae Pseudorhyncus sp. 9.3 2.7 Siemann et al. 1996 herbivore Coleoptera Curculionidae Sitona hispidula 10.4 4.0 Siemann et al. 1996 herbivore Coleoptera Elateridae genus sp. 12.3 17.5 Siemann and Elser unpublished herbivore Coleoptera Mordellidae Mordellistena sp. 9.7 2.1 Siemann et al. 1996 herbivore Coleoptera Scarabaeidae Hoplia modesta 12.3 6.8 Siemann et al. 1996 herbivore Coleoptera Scarabaeidae Phyllophaga sp. 10.4 20.0 Siemann and Elser unpublished herbivore Coleoptera Tenebrionidae Tenebrio molitor 8.0 14.5 Barker et al. 1998 herbivore Panorpida Bibionidae Bibio sp. 10.8 5.7 Siemann et al. 1996 herbivore Panorpida Chloropidae Oscinella sp. 9.3 2.2 Siemann et al. 1996 herbivore Panorpida Chloropidae Oscinella pube 9.7 4.2 Siemann et al. 1996 herbivore Panorpida Drosophilidae Drosophila arizonae 8.8 4.1 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila hydei 7.7 3.5 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila mojavensis 6.7 3.3 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila nigrospiracula 7.9 3.5 Markow et al. -

Occurrence of Ditylenchus Destructorthorne, 1945 on a Sand
Journal of Plant Protection Research ISSN 1427-4345 ORIGINAL ARTICLE Occurrence of Ditylenchus destructor Thorne, 1945 on a sand dune of the Baltic Sea Renata Dobosz1*, Katarzyna Rybarczyk-Mydłowska2, Grażyna Winiszewska2 1 Entomology and Animal Pests, Institute of Plant Protection – National Research Institute, Poznan, Poland 2 Nematological Diagnostic and Training Centre, Museum and Institute of Zoology Polish Academy of Sciences, Warsaw, Poland Vol. 60, No. 1: 31–40, 2020 Abstract DOI: 10.24425/jppr.2020.132206 Ditylenchus destructor is a serious pest of numerous economically important plants world- wide. The population of this nematode species was isolated from the root zone of Ammo- Received: July 11, 2019 phila arenaria on a Baltic Sea sand dune. This population’s morphological and morphomet- Accepted: September 27, 2019 rical characteristics corresponded to D. destructor data provided so far, except for the stylet knobs’ height (2.1–2.9 vs 1.3–1.8) and their arrangement (laterally vs slightly posteriorly *Corresponding address: sloping), the length of a hyaline part on the tail end (0.8–1.8 vs 1–2.9), the pharyngeal gland [email protected] arrangement in relation to the intestine (dorsal or ventral vs dorsal, ventral or lateral) and the appearance of vulval lips (smooth vs annulated). Ribosomal DNA sequence analysis confirmed the identity of D. destructor from a coastal dune. Keywords: Ammophila arenaria, internal transcribed spacer (ITS), potato rot nematode, 18S, 28S rDNA Introduction Nematodes from the genus Ditylenchus Filipjev, 1936, arachis Zhang et al., 2014, both of which are pests of are found in soil, in the root zone of arable and wild- peanut (Arachis hypogaea L.), Ditylenchus destruc- -growing plants, and occasionally in the tissues of un- tor Thorne, 1945 which feeds on potato (Solanum tu- derground or aboveground parts (Brzeski 1998). -

The Complete Mitochondrial Genome of the Columbia Lance Nematode
Ma et al. Parasites Vectors (2020) 13:321 https://doi.org/10.1186/s13071-020-04187-y Parasites & Vectors RESEARCH Open Access The complete mitochondrial genome of the Columbia lance nematode, Hoplolaimus columbus, a major agricultural pathogen in North America Xinyuan Ma1, Paula Agudelo1, Vincent P. Richards2 and J. Antonio Baeza2,3,4* Abstract Background: The plant-parasitic nematode Hoplolaimus columbus is a pathogen that uses a wide range of hosts and causes substantial yield loss in agricultural felds in North America. This study describes, for the frst time, the complete mitochondrial genome of H. columbus from South Carolina, USA. Methods: The mitogenome of H. columbus was assembled from Illumina 300 bp pair-end reads. It was annotated and compared to other published mitogenomes of plant-parasitic nematodes in the superfamily Tylenchoidea. The phylogenetic relationships between H. columbus and other 6 genera of plant-parasitic nematodes were examined using protein-coding genes (PCGs). Results: The mitogenome of H. columbus is a circular AT-rich DNA molecule 25,228 bp in length. The annotation result comprises 12 PCGs, 2 ribosomal RNA genes, and 19 transfer RNA genes. No atp8 gene was found in the mitog- enome of H. columbus but long non-coding regions were observed in agreement to that reported for other plant- parasitic nematodes. The mitogenomic phylogeny of plant-parasitic nematodes in the superfamily Tylenchoidea agreed with previous molecular phylogenies. Mitochondrial gene synteny in H. columbus was unique but similar to that reported for other closely related species. Conclusions: The mitogenome of H. columbus is unique within the superfamily Tylenchoidea but exhibits similarities in both gene content and synteny to other closely related nematodes.