Arthropod Parasites in Domestic Animals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolutionary History of Stomach Bot Flies in the Light of Mitogenomics
Evolutionary history of stomach bot flies in the light of mitogenomics Yan, Liping; Pape, Thomas; Elgar, Mark A.; Gao, Yunyun; Zhang, Dong Published in: Systematic Entomology DOI: 10.1111/syen.12356 Publication date: 2019 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Yan, L., Pape, T., Elgar, M. A., Gao, Y., & Zhang, D. (2019). Evolutionary history of stomach bot flies in the light of mitogenomics. Systematic Entomology, 44(4), 797-809. https://doi.org/10.1111/syen.12356 Download date: 28. Sep. 2021 Systematic Entomology (2019), 44, 797–809 DOI: 10.1111/syen.12356 Evolutionary history of stomach bot flies in the light of mitogenomics LIPING YAN1, THOMAS PAPE2 , MARK A. ELGAR3, YUNYUN GAO1 andDONG ZHANG1 1School of Nature Conservation, Beijing Forestry University, Beijing, China, 2Natural History Museum of Denmark, University of Copenhagen, Copenhagen, Denmark and 3School of BioSciences, University of Melbourne, Melbourne, Australia Abstract. Stomach bot flies (Calyptratae: Oestridae, Gasterophilinae) are obligate endoparasitoids of Proboscidea (i.e. elephants), Rhinocerotidae (i.e. rhinos) and Equidae (i.e. horses and zebras, etc.), with their larvae developing in the digestive tract of hosts with very strong host specificity. They represent an extremely unusual diver- sity among dipteran, or even insect parasites in general, and therefore provide sig- nificant insights into the evolution of parasitism. The phylogeny of stomach botflies was reconstructed -

Pest Management News
Pest Management News Dr. John D. Hopkins, Associate Professor and Extension Entomologist – Coeditor Dr. Kelly M. Loftin, Professor and Extension Entomologist – Coeditor Contributors Dr. Becky McPeake, Professor and Wildlife Extension Specialist Sherrie E. Smith, Plant Pathology Instructor, Plant Health Clinic Diagnostician Letter #6 October 31, 2017 ________________________________________________________________________________ Stopping Occasional Arthropod Invaders John D. Hopkins When the weather begins to change in the fall and things get cooler, arthropod pests like the multi-colored Asian lady beetle, the boxelder bug, crickets, various stinkbugs, or spiders are just some of the pest problems that homeowners may have to deal with. The first thing most people think of when trying to prevent a pest problem is WHAT INSECTICIDE DO I SPRAY? However, there are other measures that should be taken that will help prevent these pests from entering your home and may even eliminate the need for an insecticide application. Pest proofing your home is the BEST way to prevent unwanted invaders at this time or any other time of year. Your goal is to prevent pest entry and eliminate conditions that are conducive to pest infestation. Here are the ABC’s of pest proofing your home: A. Ensure that screens on doors and windows are properly installed and maintained. If you don't have screen doors on your home, install them. Any damaged screens should be repaired or replaced. Fine mesh screening will prevent all but the tiniest insects from entering your home. B. Doors should seal properly. If air can pass through or light can be seen through cracks around doors then insects or spiders can get in. -

First Record & Clinical Management of Tick Infestation by Amblyomma
Int. J. Adv. Res. Biol. Sci. (2020). 7(5): 71-74 International Journal of Advanced Research in Biological Sciences ISSN: 2348-8069 www.ijarbs.com DOI: 10.22192/ijarbs Coden: IJARQG (USA) Volume 7, Issue 5 -2020 Short Communication DOI: http://dx.doi.org/10.22192/ijarbs.2020.07.05.009 First Record & Clinical Management of Tick Infestation by Amblyomma gervaisi, Giardiasis and Tail Injury in a Bengal Monitor (Varanus bengalensis; Daudin, 1802) in Himmatnagar, Gujarat (India) C. M. Bhadesiya*, V. A. Patel, P. J. Gajjar and M. J. Anikar Postgraduate Institute of Veterinary Education & Research (PGIVER), Kamdhenu University, Rajpur (Nava), Himmatnagar - 383010, Gujarat (India) *Corresponding author: [email protected] Abstract A Bengal monitor (Varanus bengalensis; Daudin, 1802) was rescued from a house near Rajpur village of Himmatnagar, Sabarkantha district, Gujarat (India) and brought to the Veterinary Hospital of Kamdhenu University at Rajpur for physical checkup before release. Physical examination revealed minor injury on tail and clinical tick infestation. Ticks were identified as Amblyomma gervaisi while excreta revealed presence of Giardia spp.. The present paper is the first record of Amblyomma gervaisi tick, giardiasis and tail injury in a Bengal monitor in Himmatnagar, Gujarat which will provide baseline information for future research. Keywords: Bengal monitor, Tick, Amblyomma gervaisi, Giardiasis, Gujarat Introduction The Bengal monitor (Varanus bengalensis; Daudin, veterinary case studies in different areas. Some 1802) or a ‘Common Indian Monitor’ is generally relevant publications include [1] Report on Aponomma found in Indian subcontinent including most of the gervaisi as a reptile parasite in Pakistan and India by states. It is included under the ‘Least Concern’ Auffenberg and Auffenberg (1990); [2] Aponomma category by the International Union for Conservation gibsoni tick infestation in monitor lizard at Nagpur by of Nature (IUCN) but the population trend is shown to Harkare et al. -
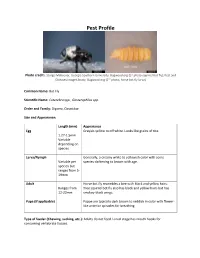
Bot Fly); Pest and Diseases Image Library, Bugwood.Org (2Nd Photo, Horse Bot Fly Larva)
Pest Profile Photo credit: Sturgis McKeever, Georgia Southern University, Bugwood.org (1st photo-squirrel bot fly); Pest and Diseases Image Library, Bugwood.org (2nd photo, horse bot fly larva) Common Name: Bot Fly Scientific Name: Cuterebra spp., Gasterophilus spp. Order and Family: Diptera, Oestridae Size and Appearance: Length (mm) Appearance Egg Grayish-yellow to off white. Looks like grains of rice. 1.27-1.5mm Variable depending on species Larva/Nymph Generally, a creamy white to yellowish color with some Variable per species darkening to brown with age. species but ranges from 5- 19mm Adult Horse bot fly resembles a bee with black and yellow hairs. Ranges from Tree squirrel bot fly also has black and yellow hairs but has 12-22mm smokey-black wings. Pupa (if applicable) Pupae are typically dark brown to reddish in color with flower- like anterior spiracles for breathing. Type of feeder (Chewing, sucking, etc.): Adults do not feed. Larval stage has mouth hooks for consuming vertebrate tissues. Hosts: The larval stages are obligate parasites that feed on the living tissues of their mammalian hosts. Horse bot flies tend to target horses but will also invade other equine species such as donkeys and mules. Tree squirrel bot flies feed on eastern gray squirrels, fox squirrels, and chipmunks. Description of Damage (larvae and adults): Tree squirrel bots are generally of no consequence other than the possibility of accidental myiasis in a cat or dog. Horse bot flies can cause irritation and stress in horses they infect. When the larvae invade the gastrointestinal tract, severe complications can arise such as colic, blockages, and other ulcers or ruptures. -

Fleas, Hosts and Habitat: What Can We Predict About the Spread of Vector-Borne Zoonotic Diseases?
2010 Fleas, Hosts and Habitat: What can we predict about the spread of vector-borne zoonotic diseases? Ph.D. Dissertation Megan M. Friggens School of Forestry I I I \, l " FLEAS, HOSTS AND HABITAT: WHAT CAN WE PREDICT ABOUT THE SPREAD OF VECTOR-BORNE ZOONOTIC DISEASES? by Megan M. Friggens A Dissertation Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Forest Science Northern Arizona University May 2010 ?Jii@~-~-u-_- Robert R. Parmenter, Ph. D. ~",l(*~ l.~ Paulette L. Ford, Ph. D. --=z:r-J'l1jU~ David M. Wagner, Ph. D. ABSTRACT FLEAS, HOSTS AND HABITAT: WHAT CAN WE PREDICT ABOUT THE SPREAD OF VECTOR-BORNE ZOONOTIC DISEASES? MEGAN M. FRIGGENS Vector-borne diseases of humans and wildlife are experiencing resurgence across the globe. I examine the dynamics of flea borne diseases through a comparative analysis of flea literature and analyses of field data collected from three sites in New Mexico: The Sevilleta National Wildlife Refuge, the Sandia Mountains and the Valles Caldera National Preserve (VCNP). My objectives were to use these analyses to better predict and manage for the spread of diseases such as plague (Yersinia pestis). To assess the impact of anthropogenic disturbance on flea communities, I compiled and analyzed data from 63 published empirical studies. Anthropogenic disturbance is associated with conditions conducive to increased transmission of flea-borne diseases. Most measures of flea infestation increased with increasing disturbance or peaked at intermediate levels of disturbance. Future trends of habitat and climate change will probably favor the spread of flea-borne disease. -

Plasma Pharmacokinetic Profile of Fluralaner (Bravecto™) and Ivermectin Following Concurrent Administration to Dogs Feli M
Walther et al. Parasites & Vectors (2015) 8:508 DOI 10.1186/s13071-015-1123-8 SHORT REPORT Open Access Plasma pharmacokinetic profile of fluralaner (Bravecto™) and ivermectin following concurrent administration to dogs Feli M. Walther1*, Mark J. Allan2 and Rainer KA Roepke2 Abstract Background: Fluralaner is a novel systemic ectoparasiticide for dogs providing immediate and persistent flea, tick and mite control after a single oral dose. Ivermectin has been used in dogs for heartworm prevention and at off label doses for mite and worm infestations. Ivermectin pharmacokinetics can be influenced by substances affecting the p-glycoprotein transporter, potentially increasing the risk of ivermectin neurotoxicity. This study investigated ivermectin blood plasma pharmacokinetics following concurrent administration with fluralaner. Findings: Ten Beagle dogs each received a single oral administration of either 56 mg fluralaner (Bravecto™), 0.3 mg ivermectin or 56 mg fluralaner plus 0.3 mg ivermectin/kg body weight. Blood plasma samples were collected at multiple post-treatment time points over a 12-week period for fluralaner and ivermectin plasma concentration analysis. Ivermectin blood plasma concentration profile and pharmacokinetic parameters Cmax,tmax,AUC∞ and t½ were similar in dogs administered ivermectin only and in dogs administered ivermectin concurrently with fluralaner, and the same was true for fluralaner pharmacokinetic parameters. Conclusions: Concurrent administration of fluralaner and ivermectin does not alter the pharmacokinetics -

Zoology Addition to the Mite Fauna in Human Habitation from South
Volume : 5 | Issue : 7 | July 2016 • ISSN No 2277 - 8179 | IF : 3.508 | IC Value : 69.48 Original Research Paper Original Research Paper Volume : 5 | Issue : 7 | July 2016 • ISSN No 2277 - 8179 | IF : 3.508 | IC Value : 69.48 Zoology Addition To The Mite Fauna in Human KEYWORDS : Human habitation, Prostigmata, Mesostigmata, Astigmata, Habitation From South Bengal South Bengal Post Graduate Department of Zoology, Vidyasagar College, Salt Lake City, CL Ananya Das Block, Kolkata 700 091 Post Graduate Department of Zoology, Vidyasagar College, Salt Lake City, CL S.K. Gupta Block, Kolkata 700 091 Post Graduate Department of Zoology, Vidyasagar College, Salt Lake City, CL N. Debnath Block, Kolkata 700 091 ABSTRACT The present paper reports the occurrence of 111 species of mites belonging to 69 genera,27 families under 3 orders collected from a total of 40 samples representing 5 different habitats viz. stored products, house dust, bird nests, cattle sheds and roof gardens from 5 districts of South Bengal. Among the 5 habitats, cattle shed provided richest diversity both in respect of species and genera followed by stored product habitat and the minimum was bird nest which represented only 11 species. The family level diversity was also highest in case of cattle sheds followed by stored products and the minimum was in roof garden. There was not a single species which could be collected from all the 5 habitats though; of course, there was 1 species which represented 4 out of 5 habitats. Therefore, cattle sheds proved to be habitat showing highest diversity. The order Prostigmata represented highest number of species followed by Astigmata. -

Ectoparasites of Free-Roaming Domestic Cats in the Central United States
Veterinary Parasitology 228 (2016) 17–22 Contents lists available at ScienceDirect Veterinary Parasitology journal homepage: www.elsevier.com/locate/vetpar Research paper Ectoparasites of free-roaming domestic cats in the central United States a b,1 a a,∗ Jennifer E. Thomas , Lesa Staubus , Jaime L. Goolsby , Mason V. Reichard a Department of Veterinary Pathobiology, Center for Veterinary Health Sciences, Oklahoma State University, 250 McElroy Hall Stillwater, OK 74078, USA b Department of Clinical Science, Center for Veterinary Health Sciences, Oklahoma State University, 1 Boren Veterinary Medical Teaching Hospital Stillwater, OK 74078, USA a r t i c l e i n f o a b s t r a c t Article history: Free-roaming domestic cat (Felis catus) populations serve as a valuable resource for studying ectoparasite Received 11 May 2016 prevalence. While they share a similar environment as owned cats, free-roaming cats do not receive rou- Received in revised form 27 July 2016 tine veterinary care or ectoparasiticide application, giving insight into parasite risks for owned animals. Accepted 29 July 2016 We examined up to 673 infested cats presented to a trap-neuter-return (TNR) clinic in the central United States. Ectoparasite prevalences on cats were as follows: fleas (71.6%), ticks (18.7%), Felicola subrostratus Keywords: (1.0%), Cheyletiella blakei (0.9%), and Otodectes cynotis (19.3%). Fleas, ticks, and O. cynotis were found in Cat all months sampled. A total of 1117 fleas were recovered from 322 infested cats. The predominate flea Feline recovered from cats was Ctenocephalides felis (97.2%) followed by Pulex spp. -

Fleas and Flea-Borne Diseases
International Journal of Infectious Diseases 14 (2010) e667–e676 Contents lists available at ScienceDirect International Journal of Infectious Diseases journal homepage: www.elsevier.com/locate/ijid Review Fleas and flea-borne diseases Idir Bitam a, Katharina Dittmar b, Philippe Parola a, Michael F. Whiting c, Didier Raoult a,* a Unite´ de Recherche en Maladies Infectieuses Tropicales Emergentes, CNRS-IRD UMR 6236, Faculte´ de Me´decine, Universite´ de la Me´diterrane´e, 27 Bd Jean Moulin, 13385 Marseille Cedex 5, France b Department of Biological Sciences, SUNY at Buffalo, Buffalo, NY, USA c Department of Biology, Brigham Young University, Provo, Utah, USA ARTICLE INFO SUMMARY Article history: Flea-borne infections are emerging or re-emerging throughout the world, and their incidence is on the Received 3 February 2009 rise. Furthermore, their distribution and that of their vectors is shifting and expanding. This publication Received in revised form 2 June 2009 reviews general flea biology and the distribution of the flea-borne diseases of public health importance Accepted 4 November 2009 throughout the world, their principal flea vectors, and the extent of their public health burden. Such an Corresponding Editor: William Cameron, overall review is necessary to understand the importance of this group of infections and the resources Ottawa, Canada that must be allocated to their control by public health authorities to ensure their timely diagnosis and treatment. Keywords: ß 2010 International Society for Infectious Diseases. Published by Elsevier Ltd. All rights reserved. Flea Siphonaptera Plague Yersinia pestis Rickettsia Bartonella Introduction to 16 families and 238 genera have been described, but only a minority is synanthropic, that is they live in close association with The past decades have seen a dramatic change in the geographic humans (Table 1).4,5 and host ranges of many vector-borne pathogens, and their diseases. -

Epidemiology of Crimean-Congo Hemorrhagic Fever in Senegal: Temporal and Spatial Patterns
Arch Virol (1990) [Suppl I]: 323-340 0 by Springer-Verlag 1990 Epidemiology of Crimean-Congo hemorrhagic fever in Senegal: temporal and spatial patterns M. L. Wilson''2, J.-P. G>zale~l'~, B. LeGuenno', J.-P. Cornet3, M. Guillaud4, M.-A. Caívo', J.-P. Digoutte', and J.-L. Camicas' c 'Institut Pasteur, Dakar, Senegal 'Departments of Population Sciences and Tropical Public Health, Harvard School of Public Health, Boston, Massachusetts, U.S.A. 31nstitut Francais de Recherche Scientifique pour le Developpement en Cooperation (ORSTOM), Laboratoire ORSTOM de Zoologie medicale, Dakar, Senegal "Institut d'Elevage et de Medecine Veterinaire des Pays Tropicaux, Maisons Alfort, France Accepted April 15, 1990 Summary. Aspects of the spatial and temporal patterns of transmission of Crimean-Congo hemorrhagic fever (CCHF) virus were studied in Senegal, West Africa. A country-wide serological survey of domestic animals indi- cated that transmission was most intense in the northern dry sahelian zone and least in the southern, more humid guinean zone. Human IgG prevalence, ranging from nearly 20% to < 1% among 8 sites throughout the region, also was greatest in the north. A fatal human case of CCHF from Rosso, Mauritania in 1988 was studied and an accompanying serosurvey of human contacts and domestic animals indicated epidemic transmission during that period. Systematic samples of adult ixodid ticks on domestic animals allowed us to analyze the distribution and relative abundance of potential CCHF virus vectors, demonstrating that Hyalomma spp. predominated in those biotopes where transmission was most intense. A prospective study of CCHF virus infection and tick infestation in sheep exposed a period of epizootic transmission in 1988 that corresponded temporally with increased abund- ance of adult H. -

Diapause and Quiescence As Two Main Kinds of Dormancy and Their Significance in Life Cycles of Mites and Ticks (Chelicerata: Arachnida: Acari)
Acarina 17 (1): 3–32 © Acarina 2009 DIAPAUSE AND QUIESCENCE AS TWO MAIN KINDS OF DORMANCY AND THEIR SIGNIFICANCE IN LIFE CYCLES OF MITES AND TICKS (CHELICERATA: ARACHNIDA: ACARI). PART 2. PARASITIFORMES V. N. Belozerov Biological Research Institute, St. Petersburg State University, Peterhof 198504, Russia; e-mail: [email protected] ABSTRACT: Concerning the problem of life history and such an important its aspect as seasonality of life cycles and their control enabled by dormant stages, the parasitiform mites reveal the obvious similarity with the acariform mites. This concerns the pres- ence of both main kinds of dormancy (diapause and quiescence). The great importance in the seasonal control of life cycles in some parasitiform mites, like in acariform mites, belongs also for combinations of diapause with non-diapause arrests, particularly with the post-diapause quiescence (PDQ). This type of quiescence evoked after termination of diapause and enabling more accu- rate time-adjustment in recommencement of active development, is characteristic of both lineages of the Parasitiformes — Ixodida and Mesostigmata (particularly Gamasida). The available data show that in ixodid ticks the PDQ may be resulted similarly after developmental and behavioral diapause. Reproductive diapause combined with the PDQ is characteristic of some gamasid mites (particularly the family Phytoseiidae), while most gamasid and uropodid mites with phoretic dispersal reveal the dormant state (apparently of diapause nature) at the deutonymphal stage. The uncertainty between diapause and non-diapause dormancy is retained in some many cases (even in ixodid ticks and phytoseiid mites), and the necessity of further thorough study of different forms of diapause and non-diapause arrests in representatives of the Acari is noted therefore. -

Nigerian Veterinary Journal 39(3)
Nigerian Veterinary Journal 39(3). 2018 Olaosebikan et al. NIGERIAN VETERINARY JOURNAL ISSN 0331-3026 Nig. Vet. J., September 2018 Vol 39 (3): 217 - 226. https://dx.doi.org/10.4314/nvj.v39i3.5 ORIGINAL ARTICLE Haematological Changes Associated with Porcine Haemoparasitic Infections in Ibadan, Oyo State, Nigeria Olaosebikan, O. O.; Alaka, O. O. and Ajadi, A. A.* Department of Veterinary Pathology, Faculty of Veterinary Medicine, University of Ibadan. *Corresponding author: Email: [email protected]; Tel No:+2349061959556 SUMMARY The study was carried out between January and July 2016. Blood samples were obtained from 153 pigs by venipuncture and jugular severance at slaughter. The blood samples were examined for all known hemoparasites detectable by light microscopic examination. Haematimetric indices, complete blood cell count and leukocyte differentials were determined. The level of parasitaemia and changes in blood indices were subjected to statistical analysis across seasons. Trypanosoma brucei and Eperythrozoon suis were the only hemoparasites detected in the blood of pigs during the period of sampling. The prevalence of haemoparasitic infections in sampled pigs was 5.23%. T. brucei contributed 3.9% while E. suis contributed 1.31% to the prevalence. Anaemia (PCV<32) was a consistent and significant finding in all parasitemic samples. Eperythrozoon suis caused more severe anaemia (20±9.89) when compared with Trypanosoma brucei (27±3.03). The anaemia caused by E. suis was mostly microcytic normochromic while T. brucei mostly caused normocytic normochromic anaemia. Mild leucopenia was observed in eperythrozoonosis while a moderate lymphocytosis was observed in T. brucei infections. It was observed that in spite of intense chemoprophylaxis and other control measures employed, we still have persistent infections with Eperythrozoon sp and Trypanosomes in our pig population.