Insecta Mundi 0165: 1-4
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insect Orders V: Panorpida & Hymenoptera
Insect Orders V: Panorpida & Hymenoptera • The Panorpida contain 5 orders: the Mecoptera, Siphonaptera, Diptera, Trichoptera and Lepidoptera. • Available evidence clearly indicates that the Lepidoptera and the Trichoptera are sister groups. • The Siphonaptera and Mecoptera are also closely related but it is not clear whether the Siponaptera is the sister group of all of the Mecoptera or a group (Boreidae) within the Mecoptera. If the latter is true, then the Mecoptera is paraphyletic as currently defined. • The Diptera is the sister group of the Siphonaptera + Mecoptera and together make up the Mecopteroids. • The Hymenoptera does not appear to be closely related to any of the other holometabolous orders. Mecoptera (Scorpionflies, hangingflies) • Classification. 600 species worldwide, arranged into 9 families (5 in the US). A very old group, many fossils from the Permian (260 mya) onward. • Structure. Most distinctive feature is the elongated clypeus and labrum that together form a rostrum. The order gets its common name from the gential segment of the male in the family Panorpodiae, which is bulbous and often curved forward above the abdomen, like the sting of a scorpion. Larvae are caterpillar-like or grub- like. • Natural history. Scorpionflies are most common in cool, moist habitats. They get the name “hangingflies” from their habit of hanging upside down on vegetation. Larvae and adult males are mostly predators or scavengers. Adult females are usually scavengers. Larvae and adults in some groups may feed on vegetation. Larvae of most species are terrestrial and caterpillar-like in body form. Larvae of some species are aquatic. In the family Bittacidae males attract females for mating by releasing a sex pheromone and then presenting the female with a nuptial gift. -
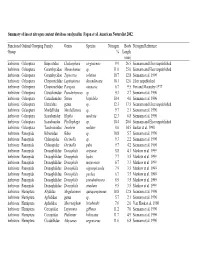
Summary of Insect Nitrogen Content Database Analyzed in Fagan Et Al
Summary of insect nitrogen content database analyzed in Fagan et al. American Naturalist 2002. Functional Ordinal Grouping Family Genus Species Nitrogen Body Nitrogen Reference Group % Length (mm) herbivore Coleoptera Buprestidae Chalcophora virginiensis 9.9 26.5 Siemann and Elser unpublished herbivore Coleoptera Cerambycidae Monochamus sp. 11.0 25.0 Siemann and Elser unpublished herbivore Coleoptera Cerambycidae Typocerus velutina 10.7 12.8 Siemann et al. 1996 herbivore Coleoptera Chrysomelidae Leptinotarsa decemlineata 10.1 12.0 Elser unpublished herbivore Coleoptera Chrysomelidae Paropsis atomaria 6.7 9.5 Fox and Macauley 1977 herbivore Coleoptera Curculionidae Pseudorhyncus sp. 9.3 2.7 Siemann et al. 1996 herbivore Coleoptera Curculionidae Sitona hispidula 10.4 4.0 Siemann et al. 1996 herbivore Coleoptera Elateridae genus sp. 12.3 17.5 Siemann and Elser unpublished herbivore Coleoptera Mordellidae Mordellistena sp. 9.7 2.1 Siemann et al. 1996 herbivore Coleoptera Scarabaeidae Hoplia modesta 12.3 6.8 Siemann et al. 1996 herbivore Coleoptera Scarabaeidae Phyllophaga sp. 10.4 20.0 Siemann and Elser unpublished herbivore Coleoptera Tenebrionidae Tenebrio molitor 8.0 14.5 Barker et al. 1998 herbivore Panorpida Bibionidae Bibio sp. 10.8 5.7 Siemann et al. 1996 herbivore Panorpida Chloropidae Oscinella sp. 9.3 2.2 Siemann et al. 1996 herbivore Panorpida Chloropidae Oscinella pube 9.7 4.2 Siemann et al. 1996 herbivore Panorpida Drosophilidae Drosophila arizonae 8.8 4.1 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila hydei 7.7 3.5 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila mojavensis 6.7 3.3 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila nigrospiracula 7.9 3.5 Markow et al. -

About the Book the Format Acknowledgments
About the Book For more than ten years I have been working on a book on bryophyte ecology and was joined by Heinjo During, who has been very helpful in critiquing multiple versions of the chapters. But as the book progressed, the field of bryophyte ecology progressed faster. No chapter ever seemed to stay finished, hence the decision to publish online. Furthermore, rather than being a textbook, it is evolving into an encyclopedia that would be at least three volumes. Having reached the age when I could retire whenever I wanted to, I no longer needed be so concerned with the publish or perish paradigm. In keeping with the sharing nature of bryologists, and the need to educate the non-bryologists about the nature and role of bryophytes in the ecosystem, it seemed my personal goals could best be accomplished by publishing online. This has several advantages for me. I can choose the format I want, I can include lots of color images, and I can post chapters or parts of chapters as I complete them and update later if I find it important. Throughout the book I have posed questions. I have even attempt to offer hypotheses for many of these. It is my hope that these questions and hypotheses will inspire students of all ages to attempt to answer these. Some are simple and could even be done by elementary school children. Others are suitable for undergraduate projects. And some will take lifelong work or a large team of researchers around the world. Have fun with them! The Format The decision to publish Bryophyte Ecology as an ebook occurred after I had a publisher, and I am sure I have not thought of all the complexities of publishing as I complete things, rather than in the order of the planned organization. -

Fleas (Siphonaptera) Are Cretaceous, and Evolved with Theria
bioRxiv preprint doi: https://doi.org/10.1101/014308; this version posted January 24, 2015. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Fleas (Siphonaptera) are Cretaceous, and Evolved with Theria Qiyun Zhu1, Michael Hastriter2, Michael Whiting2, 3, Katharina Dittmar1, 4* Jan. 23, 2015 Abstract: Fleas (order Siphonaptera) are highly-specialized, diverse blood-feeding ectoparasites of mammals and birds with an enigmatic evolutionary history and obscure origin. We here present a molecular phylogenetic study based on a compre- hensive taxon sampling of 259 flea taxa, representing 16 of the 18 extant families of this order. A Bayesian phylogenetic tree with strong nodal support was recovered, consisting of seven sequentially derived lineages with Macropsyllidae at the base and Stephanocircidae as the second basal group. Divergence times of flea lineages were estimated based on fossil records and host specific associations to bats (Chiroptera), showing that the common ancestor of extant Siphonaptera split from its clos- est mecopteran sister group in the Early Cretaceous and basal lineages diversified during the Late Cretaceous. However, most of the intraordinal divergence into families took place after the K-Pg boundary. Ancestral states of host association and bioge- ographical distribution were reconstructed, suggesting with high likelihood that fleas originated in the southern continents (Gondwana) and migrated from South America to their extant distributions in a relatively short time frame. Theria (placental mammals and marsupials) represent the most likely ancestral host group of extant Siphonaptera, with marsupials occupying a more important role than previously assumed. -

Mecoptera: Meropeidae): Simply Dull Or Just Inscrutable?
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Center for Systematic Entomology, Gainesville, Insecta Mundi Florida 8-24-2007 Etymology of the earwigfly, Merope tuber Newman (Mecoptera: Meropeidae): Simply dull or just inscrutable? Louis A. Somma University of Florida, [email protected] James C. Dunford University of Florida, Gainesville, FL Follow this and additional works at: https://digitalcommons.unl.edu/insectamundi Part of the Entomology Commons Somma, Louis A. and Dunford, James C., "Etymology of the earwigfly, Merope tuber Newman (Mecoptera: Meropeidae): Simply dull or just inscrutable?" (2007). Insecta Mundi. 65. https://digitalcommons.unl.edu/insectamundi/65 This Article is brought to you for free and open access by the Center for Systematic Entomology, Gainesville, Florida at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Insecta Mundi by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. INSECTA MUNDI A Journal of World Insect Systematics 0013 Etymology of the earwigfly, Merope tuber Newman (Mecoptera: Meropeidae): Simply dull or just inscrutable? Louis A. Somma Department of Zoology PO Box 118525 University of Florida Gainesville, FL 32611-8525 [email protected] James C. Dunford Department of Entomology and Nematology PO Box 110620, IFAS University of Florida Gainesville, FL 32611-0620 [email protected] Date of Issue: August 24, 2007 CENTER FOR SYSTEMATIC ENTOMOLOGY, INC., Gainesville, FL Louis A. Somma and James C. Dunford Etymology of the earwigfly, Merope tuber Newman (Mecoptera: Meropeidae): Simply dull or just inscrutable? Insecta Mundi 0013: 1-5 Published in 2007 by Center for Systematic Entomology, Inc. P. O. Box 147100 Gainesville, FL 32604-7100 U. -

Allometric and Phylogenetic Variation in Insect Phosphorus 18, 103–109 Content
Functional Blackwell Publishing, Ltd. Ecology 2004 Allometric and phylogenetic variation in insect phosphorus 18, 103–109 content H. A. WOODS,*† W. F. FAGAN,‡ J. J. ELSER§ and J. F. HARRISON§ *Section of Integrative Biology C0930, University of Texas at Austin, Austin, TX 78712, USA, ‡Department of Biology, University of Maryland, College Park, MD 20742, USA, and §Department of Biology, Arizona State University, Tempe, AZ 85287–1501, USA Summary 1. Phosphorus content was measured in adult insects and arachnids from 170 species collected in the Sonoran Desert. 2. Across insect body sizes spanning four orders of magnitude, phosphorus content was inversely related to body mass. The largest species (∼1 g dry) had phosphorus contents that were only about 60% (0·62% P absolute) as high as phosphorus contents of the smallest species (∼0·0001 g dry; 0·97% P). Negative phosphorus allometry was observed within each of seven insect orders and within arachnids. 3. Phosphorus contents of insect predators and herbivores were statistically indistinguishable. 4. More recently derived orders tended to have lower phosphorus contents – with the exception of the most recently derived group (Panorpida = Diptera + Lepidoptera), which had high phosphorus contents. Key-words: Allometry, body size, exoskeleton, phosphorus limitation, stoichiometry Functional Ecology (2004) 18, 103–109 processes dependent on elemental composition (e.g. Introduction nutrient cycling) and because P occupies a key position Herbivorous insects face a fundamental asymmetry in the recently developed theory of ecological stoichio- between the nutrient contents of their body tissues and metry (Sterner & Elser 2002). those of the plants they eat (Slansky & Feeny 1977; Several expectations can be derived a priori by McNeill & Southwood 1978; Mattson 1980; Strong, extensions of previous work on N. -

Phylogeny of Endopterygote Insects, the Most Successful Lineage of Living Organisms*
REVIEW Eur. J. Entomol. 96: 237-253, 1999 ISSN 1210-5759 Phylogeny of endopterygote insects, the most successful lineage of living organisms* N iels P. KRISTENSEN Zoological Museum, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen 0, Denmark; e-mail: [email protected] Key words. Insecta, Endopterygota, Holometabola, phylogeny, diversification modes, Megaloptera, Raphidioptera, Neuroptera, Coleóptera, Strepsiptera, Díptera, Mecoptera, Siphonaptera, Trichoptera, Lepidoptera, Hymenoptera Abstract. The monophyly of the Endopterygota is supported primarily by the specialized larva without external wing buds and with degradable eyes, as well as by the quiescence of the last immature (pupal) stage; a specialized morphology of the latter is not an en dopterygote groundplan trait. There is weak support for the basal endopterygote splitting event being between a Neuropterida + Co leóptera clade and a Mecopterida + Hymenoptera clade; a fully sclerotized sitophore plate in the adult is a newly recognized possible groundplan autapomorphy of the latter. The molecular evidence for a Strepsiptera + Díptera clade is differently interpreted by advo cates of parsimony and maximum likelihood analyses of sequence data, and the morphological evidence for the monophyly of this clade is ambiguous. The basal diversification patterns within the principal endopterygote clades (“orders”) are succinctly reviewed. The truly species-rich clades are almost consistently quite subordinate. The identification of “key innovations” promoting evolution -

2015-2025 Pennsylvania Wildlife Action Plan
2 0 1 5 – 2 0 2 5 Species Assessments Appendix 1.1A – Birds A Comprehensive Status Assessment of Pennsylvania’s Avifauna for Application to the State Wildlife Action Plan Update 2015 (Jason Hill, PhD) Assessment of eBird data for the importance of Pennsylvania as a bird migratory corridor (Andy Wilson, PhD) Appendix 1.1B – Mammals A Comprehensive Status Assessment of Pennsylvania’s Mammals, Utilizing NatureServe Ranking Methodology and Rank Calculator Version 3.1 for Application to the State Wildlife Action Plan Update 2015 (Charlie Eichelberger and Joe Wisgo) Appendix 1.1C – Reptiles and Amphibians A Revision of the State Conservation Ranks of Pennsylvania’s Herpetofauna Appendix 1.1D – Fishes A Revision of the State Conservation Ranks of Pennsylvania’s Fishes Appendix 1.1E – Invertebrates Invertebrate Assessment for the 2015 Pennsylvania Wildlife Action Plan Revision 2015-2025 Pennsylvania Wildlife Action Plan Appendix 1.1A - Birds A Comprehensive Status Assessment of Pennsylvania’s Avifauna for Application to the State Wildlife Action Plan Update 2015 Jason M. Hill, PhD. Table of Contents Assessment ............................................................................................................................................. 3 Data Sources ....................................................................................................................................... 3 Species Selection ................................................................................................................................ -

A New Earwigfly from Mid-Cretaceous Burmese Amber (Mecoptera
Cretaceous Research 66 (2016) 136e140 Contents lists available at ScienceDirect Cretaceous Research journal homepage: www.elsevier.com/locate/CretRes Short communication A new earwigfly from mid-Cretaceous Burmese amber (Mecoptera: Meropeidae) ** Xiangdong Zhao a, b, Qingqing Zhang b, c, Edmund A. Jarzembowski b, d, Lei Chen a, b, , * Bo Wang b, e, a Shandong University of Science and Technology, Qingdao 266510, PR China b State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing 210008, PR China c University of the Chinese Academy of Sciences, Beijing 100049, PR China d Department of Earth Sciences, Natural History Museum, London SW7 5BD, UK e Key Laboratory of Zoological Systematics and Evolution, Institute of Zoology, Chinese Academy of Science, Beijing 100101, PR China article info abstract Article history: A new species of Meropeidae (earwigfly) is described and figured based on an exceptionally well- Received 24 April 2016 preserved individual in mid-Cretaceous amber from Myanmar. Burmomerope clara Zhao and Wang, sp. Received in revised form nov. is distinguished from the type species B. eureka Grimaldi and Engel, 2013 by presence of broader 13 June 2016 wings with six longitudinal veins in radial sector and seven in medial field, CuA with two terminal Accepted in revised form 14 June 2016 branches, and long setae on the anterior margin of the wing. A detailed comparison of the forewings Available online 16 June 2016 venation in all fossil and extant species is given. The new find is the third fossil species of Meropeidae and also the first fossil female to be described. -

New Species of Neopanorpa (Mecoptera) from Vietnam, with a Key to the Species of Mecoptera of Vietnam Author(S): Wesley J
New Species of Neopanorpa (Mecoptera) from Vietnam, with a Key to the Species of Mecoptera of Vietnam Author(s): Wesley J. Bicha, Nathan Schiff, Thai Hong Pham, Aaron Lancaster and Brian Scheffler Source: Proceedings of the Entomological Society of Washington, 119(4):529-544. Published By: Entomological Society of Washington https://doi.org/10.4289/0013-8797.119.4.529 URL: http://www.bioone.org/doi/full/10.4289/0013-8797.119.4.529 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/ terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. PROC. ENTOMOL. SOC. WASH. 119(4), 2017, pp. 529–544 NEW SPECIES OF NEOPANORPA (MECOPTERA) FROM VIETNAM, WITH A KEY TO THE SPECIES OF MECOPTERA OF VIETNAM urn:lsid:zoobank.org:pub:CE24D561-5F0E-482F-953D-B0B809D4A607 WESLEY J. BICHA,NATHAN SCHIFF,THAI HONG PHAM,AARON LANCASTER, AND BRIAN SCHEFFLER (WJB) Tropical Research Associates Entomology, 521 46th Street, Sacramento, California 95819 (e-mail: [email protected]); (NS) United States Department of Agriculture Forest Service, Southern Research Station, Center for Bottomland Hardwoods Research, P.O. -

Ceny Bursztynu Bałtyckiego If Not Amber, Then What? Fakes at the IAA Amber Laboratory in 2016 13 Amber Prices Mody(Fikacje) Bursztynu Nowelizacja Prawa Geologicznego
/ Gdańsk / Poland / / AMBEREXPO / 2017 ambermart 18th International Amber Fair 31.08–02.09.2017 ambermart.pl 2018 amberif 25th International Fair of Amber, Jewellery and Gemstones 21–24.03.2018 amberif.pl jewellery by Jola & Andrzej Kupniewscy fashion by Pudu Joanna Weyna hat by Beata Miłogrodzka SPIS TREŚCI | TABLE OF CONTENTS / Gdańsk / Poland / / AMBEREXPO / LUDZIE BURSZTYNU | AMBER PERSONALITIES PROMOCJA BURSZTYNU | AMBER PROMOTION Bursztynnik Roku 2016 - Zoja Kostiaszowa S&A i najpiękniejsze Polki 4 Amber Personality of the Year 2016 - Zoja Kostiashova 36 S&A and the most beautiful Polish women DIAGNOSTYKA BURSZTYNU | AMBER DIAGNOSTIC Wiesław GierłowskI - Bursztynnik Stulecia. Wspomnienie 6 Amber personality of all the time. Posthumous tribute Gemmologiczne badania nad bursztynem i innymi żywicami kopalnymi w Państwowym Centrum Gemmologicznym Ukrainy RYNEK BURSZTYNU | AMBER MARKET 38 Gemological study of amber and other fossil resins in State Gemological Center of Ukraine Norma bursztynowa – zakończenie prac nad projektem 12 The Amber Standard—work ends on draft Jeśli nie bursztyn to co? Imitacje w Laboratorium Bursztynu MSB 42 w 2016 roku Ceny bursztynu bałtyckiego If not amber, then what? Fakes at the IAA Amber Laboratory in 2016 13 Amber prices Mody(fikacje) bursztynu Nowelizacja prawa geologicznego. Stanowisko MSB 44 Fashioning Amber 14 New version of the Polish Geology Law. The IAA’s position WYDARZENIA | EVENTS 16 MSB na targach w 2016 IAA at a trade fairs in 2016 46 The Fullmoon of GEMUnity (GIT2016) Piąta Międzynarodowa Konferencja -

Fleas Are Parasitic Scorpionflies
Palaeoentomology 003 (6): 641–653 ISSN 2624-2826 (print edition) https://www.mapress.com/j/pe/ PALAEOENTOMOLOGY PE Copyright © 2020 Magnolia Press Article ISSN 2624-2834 (online edition) https://doi.org/10.11646/palaeoentomology.3.6.16 http://zoobank.org/urn:lsid:zoobank.org:pub:9B7B23CF-5A1E-44EB-A1D4-59DDBF321938 Fleas are parasitic scorpionflies ERIK TIHELKA1, MATTIA GIACOMELLI1, 2, DI-YING HUANG3, DAVIDE PISANI1, 2, PHILIP C. J. DONOGHUE1 & CHEN-YANG CAI3, 1, * 1School of Earth Sciences University of Bristol, Life Sciences Building, Tyndall Avenue, Bristol, BS8 1TQ, UK 2School of Life Sciences University of Bristol, Life Sciences Building, Tyndall Avenue, Bristol, BS8 1TQ, UK 3State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, and Centre for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, Nanjing 210008, China [email protected]; https://orcid.org/0000-0002-5048-5355 [email protected]; https://orcid.org/0000-0002-0554-3704 [email protected]; https://orcid.org/0000-0002-5637-4867 [email protected]; https://orcid.org/0000-0003-0949-6682 [email protected]; https://orcid.org/0000-0003-3116-7463 [email protected]; https://orcid.org/0000-0002-9283-8323 *Corresponding author Abstract bizarre bodyplans and modes of life among insects (Lewis, 1998). Flea monophyly is strongly supported by siphonate Fleas (Siphonaptera) are medically important blood-feeding mouthparts formed from the laciniae and labrum, strongly insects responsible for spreading pathogens such as plague, murine typhus, and myxomatosis. The peculiar morphology reduced eyes, laterally compressed wingless body, of fleas resulting from their specialised ectoparasitic and hind legs adapted for jumping (Beutel et al., 2013; lifestyle has meant that the phylogenetic position of this Medvedev, 2017).