Holometabolous Insects (Holometabola)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insect Orders V: Panorpida & Hymenoptera
Insect Orders V: Panorpida & Hymenoptera • The Panorpida contain 5 orders: the Mecoptera, Siphonaptera, Diptera, Trichoptera and Lepidoptera. • Available evidence clearly indicates that the Lepidoptera and the Trichoptera are sister groups. • The Siphonaptera and Mecoptera are also closely related but it is not clear whether the Siponaptera is the sister group of all of the Mecoptera or a group (Boreidae) within the Mecoptera. If the latter is true, then the Mecoptera is paraphyletic as currently defined. • The Diptera is the sister group of the Siphonaptera + Mecoptera and together make up the Mecopteroids. • The Hymenoptera does not appear to be closely related to any of the other holometabolous orders. Mecoptera (Scorpionflies, hangingflies) • Classification. 600 species worldwide, arranged into 9 families (5 in the US). A very old group, many fossils from the Permian (260 mya) onward. • Structure. Most distinctive feature is the elongated clypeus and labrum that together form a rostrum. The order gets its common name from the gential segment of the male in the family Panorpodiae, which is bulbous and often curved forward above the abdomen, like the sting of a scorpion. Larvae are caterpillar-like or grub- like. • Natural history. Scorpionflies are most common in cool, moist habitats. They get the name “hangingflies” from their habit of hanging upside down on vegetation. Larvae and adult males are mostly predators or scavengers. Adult females are usually scavengers. Larvae and adults in some groups may feed on vegetation. Larvae of most species are terrestrial and caterpillar-like in body form. Larvae of some species are aquatic. In the family Bittacidae males attract females for mating by releasing a sex pheromone and then presenting the female with a nuptial gift. -

African Butterfly News Can Be Downloaded Here
LATE SUMMER EDITION: JANUARY / AFRICAN FEBRUARY 2018 - 1 BUTTERFLY THE LEPIDOPTERISTS’ SOCIETY OF AFRICA NEWS LATEST NEWS Welcome to the first newsletter of 2018! I trust you all have returned safely from your December break (assuming you had one!) and are getting into the swing of 2018? With few exceptions, 2017 was a very poor year butterfly-wise, at least in South Africa. The drought continues to have a very negative impact on our hobby, but here’s hoping that 2018 will be better! Braving the Great Karoo and Noorsveld (Mark Williams) In the first week of November 2017 Jeremy Dobson and I headed off south from Egoli, at the crack of dawn, for the ‘Harde Karoo’. (Is there a ‘Soft Karoo’?) We had a very flexible plan for the six-day trip, not even having booked any overnight accommodation. We figured that finding a place to commune with Uncle Morpheus every night would not be a problem because all the kids were at school. As it turned out we did not have to spend a night trying to kip in the Pajero – my snoring would have driven Jeremy nuts ... Friday 3 November The main purpose of the trip was to survey two quadrants for the Karoo BioGaps Project. One of these was on the farm Lushof, 10 km west of Loxton, and the other was Taaiboschkloof, about 50 km south-east of Loxton. The 1 000 km drive, via Kimberley, to Loxton was accompanied by hot and windy weather. The temperature hit 38 degrees and was 33 when the sun hit the horizon at 6 pm. -
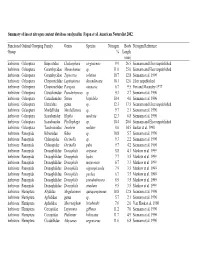
Summary of Insect Nitrogen Content Database Analyzed in Fagan Et Al
Summary of insect nitrogen content database analyzed in Fagan et al. American Naturalist 2002. Functional Ordinal Grouping Family Genus Species Nitrogen Body Nitrogen Reference Group % Length (mm) herbivore Coleoptera Buprestidae Chalcophora virginiensis 9.9 26.5 Siemann and Elser unpublished herbivore Coleoptera Cerambycidae Monochamus sp. 11.0 25.0 Siemann and Elser unpublished herbivore Coleoptera Cerambycidae Typocerus velutina 10.7 12.8 Siemann et al. 1996 herbivore Coleoptera Chrysomelidae Leptinotarsa decemlineata 10.1 12.0 Elser unpublished herbivore Coleoptera Chrysomelidae Paropsis atomaria 6.7 9.5 Fox and Macauley 1977 herbivore Coleoptera Curculionidae Pseudorhyncus sp. 9.3 2.7 Siemann et al. 1996 herbivore Coleoptera Curculionidae Sitona hispidula 10.4 4.0 Siemann et al. 1996 herbivore Coleoptera Elateridae genus sp. 12.3 17.5 Siemann and Elser unpublished herbivore Coleoptera Mordellidae Mordellistena sp. 9.7 2.1 Siemann et al. 1996 herbivore Coleoptera Scarabaeidae Hoplia modesta 12.3 6.8 Siemann et al. 1996 herbivore Coleoptera Scarabaeidae Phyllophaga sp. 10.4 20.0 Siemann and Elser unpublished herbivore Coleoptera Tenebrionidae Tenebrio molitor 8.0 14.5 Barker et al. 1998 herbivore Panorpida Bibionidae Bibio sp. 10.8 5.7 Siemann et al. 1996 herbivore Panorpida Chloropidae Oscinella sp. 9.3 2.2 Siemann et al. 1996 herbivore Panorpida Chloropidae Oscinella pube 9.7 4.2 Siemann et al. 1996 herbivore Panorpida Drosophilidae Drosophila arizonae 8.8 4.1 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila hydei 7.7 3.5 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila mojavensis 6.7 3.3 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila nigrospiracula 7.9 3.5 Markow et al. -

Fleas (Siphonaptera) Are Cretaceous, and Evolved with Theria
bioRxiv preprint doi: https://doi.org/10.1101/014308; this version posted January 24, 2015. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Fleas (Siphonaptera) are Cretaceous, and Evolved with Theria Qiyun Zhu1, Michael Hastriter2, Michael Whiting2, 3, Katharina Dittmar1, 4* Jan. 23, 2015 Abstract: Fleas (order Siphonaptera) are highly-specialized, diverse blood-feeding ectoparasites of mammals and birds with an enigmatic evolutionary history and obscure origin. We here present a molecular phylogenetic study based on a compre- hensive taxon sampling of 259 flea taxa, representing 16 of the 18 extant families of this order. A Bayesian phylogenetic tree with strong nodal support was recovered, consisting of seven sequentially derived lineages with Macropsyllidae at the base and Stephanocircidae as the second basal group. Divergence times of flea lineages were estimated based on fossil records and host specific associations to bats (Chiroptera), showing that the common ancestor of extant Siphonaptera split from its clos- est mecopteran sister group in the Early Cretaceous and basal lineages diversified during the Late Cretaceous. However, most of the intraordinal divergence into families took place after the K-Pg boundary. Ancestral states of host association and bioge- ographical distribution were reconstructed, suggesting with high likelihood that fleas originated in the southern continents (Gondwana) and migrated from South America to their extant distributions in a relatively short time frame. Theria (placental mammals and marsupials) represent the most likely ancestral host group of extant Siphonaptera, with marsupials occupying a more important role than previously assumed. -

Amphiesmeno- Ptera: the Caddisflies and Lepidoptera
CY501-C13[548-606].qxd 2/16/05 12:17 AM Page 548 quark11 27B:CY501:Chapters:Chapter-13: 13Amphiesmeno-Amphiesmenoptera: The ptera:Caddisflies The and Lepidoptera With very few exceptions the life histories of the orders Tri- from Old English traveling cadice men, who pinned bits of choptera (caddisflies)Caddisflies and Lepidoptera (moths and butter- cloth to their and coats to advertise their fabrics. A few species flies) are extremely different; the former have aquatic larvae, actually have terrestrial larvae, but even these are relegated to and the latter nearly always have terrestrial, plant-feeding wet leaf litter, so many defining features of the order concern caterpillars. Nonetheless, the close relationship of these two larval adaptations for an almost wholly aquatic lifestyle (Wig- orders hasLepidoptera essentially never been disputed and is supported gins, 1977, 1996). For example, larvae are apneustic (without by strong morphological (Kristensen, 1975, 1991), molecular spiracles) and respire through a thin, permeable cuticle, (Wheeler et al., 2001; Whiting, 2002), and paleontological evi- some of which have filamentous abdominal gills that are sim- dence. Synapomorphies linking these two orders include het- ple or intricately branched (Figure 13.3). Antennae and the erogametic females; a pair of glands on sternite V (found in tentorium of larvae are reduced, though functional signifi- Trichoptera and in basal moths); dense, long setae on the cance of these features is unknown. Larvae do not have pro- wing membrane (which are modified into scales in Lepi- legs on most abdominal segments, save for a pair of anal pro- doptera); forewing with the anal veins looping up to form a legs that have sclerotized hooks for anchoring the larva in its double “Y” configuration; larva with a fused hypopharynx case. -

Diversity and Ecosystem Services of Trichoptera
Review Diversity and Ecosystem Services of Trichoptera John C. Morse 1,*, Paul B. Frandsen 2,3, Wolfram Graf 4 and Jessica A. Thomas 5 1 Department of Plant & Environmental Sciences, Clemson University, E-143 Poole Agricultural Center, Clemson, SC 29634-0310, USA; [email protected] 2 Department of Plant & Wildlife Sciences, Brigham Young University, 701 E University Parkway Drive, Provo, UT 84602, USA; [email protected] 3 Data Science Lab, Smithsonian Institution, 600 Maryland Ave SW, Washington, D.C. 20024, USA 4 BOKU, Institute of Hydrobiology and Aquatic Ecology Management, University of Natural Resources and Life Sciences, Gregor Mendelstr. 33, A-1180 Vienna, Austria; [email protected] 5 Department of Biology, University of York, Wentworth Way, York Y010 5DD, UK; [email protected] * Correspondence: [email protected]; Tel.: +1-864-656-5049 Received: 2 February 2019; Accepted: 12 April 2019; Published: 1 May 2019 Abstract: The holometabolous insect order Trichoptera (caddisflies) includes more known species than all of the other primarily aquatic orders of insects combined. They are distributed unevenly; with the greatest number and density occurring in the Oriental Biogeographic Region and the smallest in the East Palearctic. Ecosystem services provided by Trichoptera are also very diverse and include their essential roles in food webs, in biological monitoring of water quality, as food for fish and other predators (many of which are of human concern), and as engineers that stabilize gravel bed sediment. They are especially important in capturing and using a wide variety of nutrients in many forms, transforming them for use by other organisms in freshwaters and surrounding riparian areas. -

Allometric and Phylogenetic Variation in Insect Phosphorus 18, 103–109 Content
Functional Blackwell Publishing, Ltd. Ecology 2004 Allometric and phylogenetic variation in insect phosphorus 18, 103–109 content H. A. WOODS,*† W. F. FAGAN,‡ J. J. ELSER§ and J. F. HARRISON§ *Section of Integrative Biology C0930, University of Texas at Austin, Austin, TX 78712, USA, ‡Department of Biology, University of Maryland, College Park, MD 20742, USA, and §Department of Biology, Arizona State University, Tempe, AZ 85287–1501, USA Summary 1. Phosphorus content was measured in adult insects and arachnids from 170 species collected in the Sonoran Desert. 2. Across insect body sizes spanning four orders of magnitude, phosphorus content was inversely related to body mass. The largest species (∼1 g dry) had phosphorus contents that were only about 60% (0·62% P absolute) as high as phosphorus contents of the smallest species (∼0·0001 g dry; 0·97% P). Negative phosphorus allometry was observed within each of seven insect orders and within arachnids. 3. Phosphorus contents of insect predators and herbivores were statistically indistinguishable. 4. More recently derived orders tended to have lower phosphorus contents – with the exception of the most recently derived group (Panorpida = Diptera + Lepidoptera), which had high phosphorus contents. Key-words: Allometry, body size, exoskeleton, phosphorus limitation, stoichiometry Functional Ecology (2004) 18, 103–109 processes dependent on elemental composition (e.g. Introduction nutrient cycling) and because P occupies a key position Herbivorous insects face a fundamental asymmetry in the recently developed theory of ecological stoichio- between the nutrient contents of their body tissues and metry (Sterner & Elser 2002). those of the plants they eat (Slansky & Feeny 1977; Several expectations can be derived a priori by McNeill & Southwood 1978; Mattson 1980; Strong, extensions of previous work on N. -

Phylogeny of Endopterygote Insects, the Most Successful Lineage of Living Organisms*
REVIEW Eur. J. Entomol. 96: 237-253, 1999 ISSN 1210-5759 Phylogeny of endopterygote insects, the most successful lineage of living organisms* N iels P. KRISTENSEN Zoological Museum, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen 0, Denmark; e-mail: [email protected] Key words. Insecta, Endopterygota, Holometabola, phylogeny, diversification modes, Megaloptera, Raphidioptera, Neuroptera, Coleóptera, Strepsiptera, Díptera, Mecoptera, Siphonaptera, Trichoptera, Lepidoptera, Hymenoptera Abstract. The monophyly of the Endopterygota is supported primarily by the specialized larva without external wing buds and with degradable eyes, as well as by the quiescence of the last immature (pupal) stage; a specialized morphology of the latter is not an en dopterygote groundplan trait. There is weak support for the basal endopterygote splitting event being between a Neuropterida + Co leóptera clade and a Mecopterida + Hymenoptera clade; a fully sclerotized sitophore plate in the adult is a newly recognized possible groundplan autapomorphy of the latter. The molecular evidence for a Strepsiptera + Díptera clade is differently interpreted by advo cates of parsimony and maximum likelihood analyses of sequence data, and the morphological evidence for the monophyly of this clade is ambiguous. The basal diversification patterns within the principal endopterygote clades (“orders”) are succinctly reviewed. The truly species-rich clades are almost consistently quite subordinate. The identification of “key innovations” promoting evolution -

DNA Minibarcodes in Taxonomic Assignment: a Morphologically
Zoologica Scripta DNA mini-barcodes in taxonomic assignment: a morphologically unique new homoneurous moth clade from the Indian Himalayas described in Micropterix (Lepidoptera, Micropterigidae) DAVID C. LEES,RODOLPHE ROUGERIE,CHRISTOF ZELLER-LUKASHORT &NIELS P. KRISTENSEN Submitted: 3 June 2010 Lees, D. C., Rougerie, R., Zeller-Lukashort, C. & Kristensen, N. P. (2010). DNA mini- Accepted: 24 July 2010 barcodes in taxonomic assignment: a morphologically unique new homoneurous moth doi: 10.1111/j.1463-6409.2010.00447.x clade from the Indian Himalayas described in Micropterix (Lepidoptera, Micropterigidae). — Zoologica Scripta, 39, 642–661. The first micropterigid moths recorded from the Himalayas, Micropterix cornuella sp. n. and Micropterix longicornuella sp. n. (collected, respectively, in 1935 in the Arunachel Pra- desh Province and in 1874 in Darjeeling, both Northeastern India) constitute a new clade, which is unique within the family because of striking specializations of the female postab- domen: tergum VIII ventral plate forming a continuous sclerotized ring, segment IX bear- ing a pair of strongly sclerotized lateroventral plates, each with a prominent horn-like posterior process. Fore wing vein R unforked, all Rs veins preapical; hind wing devoid of a discrete vein R. The combination of the two first-mentioned vein characters suggests close affinity to the large Palearctic genus Micropterix (to some species of which the members of the new clade bear strong superficial resemblance). Whilst absence of the hind wing R is unknown in that genus, this specialization is not incompatible with the new clade being subordinate within it. A 136-bp fragment of Cytochrome oxidase I successfully amplified from both of the 75-year-old specimens strongly supports this generic assignment. -

Insect Egg Size and Shape Evolve with Ecology but Not Developmental Rate Samuel H
ARTICLE https://doi.org/10.1038/s41586-019-1302-4 Insect egg size and shape evolve with ecology but not developmental rate Samuel H. Church1,4*, Seth Donoughe1,3,4, Bruno A. S. de Medeiros1 & Cassandra G. Extavour1,2* Over the course of evolution, organism size has diversified markedly. Changes in size are thought to have occurred because of developmental, morphological and/or ecological pressures. To perform phylogenetic tests of the potential effects of these pressures, here we generated a dataset of more than ten thousand descriptions of insect eggs, and combined these with genetic and life-history datasets. We show that, across eight orders of magnitude of variation in egg volume, the relationship between size and shape itself evolves, such that previously predicted global patterns of scaling do not adequately explain the diversity in egg shapes. We show that egg size is not correlated with developmental rate and that, for many insects, egg size is not correlated with adult body size. Instead, we find that the evolution of parasitoidism and aquatic oviposition help to explain the diversification in the size and shape of insect eggs. Our study suggests that where eggs are laid, rather than universal allometric constants, underlies the evolution of insect egg size and shape. Size is a fundamental factor in many biological processes. The size of an 526 families and every currently described extant hexapod order24 organism may affect interactions both with other organisms and with (Fig. 1a and Supplementary Fig. 1). We combined this dataset with the environment1,2, it scales with features of morphology and physi- backbone hexapod phylogenies25,26 that we enriched to include taxa ology3, and larger animals often have higher fitness4. -

New Species of Neopanorpa (Mecoptera) from Vietnam, with a Key to the Species of Mecoptera of Vietnam Author(S): Wesley J
New Species of Neopanorpa (Mecoptera) from Vietnam, with a Key to the Species of Mecoptera of Vietnam Author(s): Wesley J. Bicha, Nathan Schiff, Thai Hong Pham, Aaron Lancaster and Brian Scheffler Source: Proceedings of the Entomological Society of Washington, 119(4):529-544. Published By: Entomological Society of Washington https://doi.org/10.4289/0013-8797.119.4.529 URL: http://www.bioone.org/doi/full/10.4289/0013-8797.119.4.529 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/ terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. PROC. ENTOMOL. SOC. WASH. 119(4), 2017, pp. 529–544 NEW SPECIES OF NEOPANORPA (MECOPTERA) FROM VIETNAM, WITH A KEY TO THE SPECIES OF MECOPTERA OF VIETNAM urn:lsid:zoobank.org:pub:CE24D561-5F0E-482F-953D-B0B809D4A607 WESLEY J. BICHA,NATHAN SCHIFF,THAI HONG PHAM,AARON LANCASTER, AND BRIAN SCHEFFLER (WJB) Tropical Research Associates Entomology, 521 46th Street, Sacramento, California 95819 (e-mail: [email protected]); (NS) United States Department of Agriculture Forest Service, Southern Research Station, Center for Bottomland Hardwoods Research, P.O. -

Evolution of the Insects
CY501-C11[407-467].qxd 3/2/05 12:56 PM Page 407 quark11 Quark11:Desktop Folder:CY501-Grimaldi:Quark_files: But, for the point of wisdom, I would choose to Know the mind that stirs Between the wings of Bees and building wasps. –George Eliot, The Spanish Gypsy 11HHymenoptera:ymenoptera: Ants, Bees, and Ants,Other Wasps Bees, and The order Hymenoptera comprises one of the four “hyperdi- various times between the Late Permian and Early Triassic. verse” insectO lineages;ther the others – Diptera, Lepidoptera, Wasps and, Thus, unlike some of the basal holometabolan orders, the of course, Coleoptera – are also holometabolous. Among Hymenoptera have a relatively recent origin, first appearing holometabolans, Hymenoptera is perhaps the most difficult in the Late Triassic. Since the Triassic, the Hymenoptera have to place in a phylogenetic framework, excepting the enig- truly come into their own, having radiated extensively in the matic twisted-wings, order Strepsiptera. Hymenoptera are Jurassic, again in the Cretaceous, and again (within certain morphologically isolated among orders of Holometabola, family-level lineages) during the Tertiary. The hymenopteran consisting of a complex mixture of primitive traits and bauplan, in both structure and function, has been tremen- numerous autapomorphies, leaving little evidence to which dously successful. group they are most closely related. Present evidence indi- While the beetles today boast the largest number of cates that the Holometabola can be organized into two major species among all orders, Hymenoptera may eventually rival lineages: the Coleoptera ϩ Neuropterida and the Panorpida. or even surpass the diversity of coleopterans (Kristensen, It is to the Panorpida that the Hymenoptera appear to be 1999a; Grissell, 1999).