Genetic Diversity of Hatchery-Bred Brown Trout
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Utbygd Vannkraft Pr. 01. 01. 1990
NVE NORGES VASSDRAGS-- OG ENERGIVERK NORGES VASSDRAGS-OG ENERGIVERK BIBLIOTEK UTBYGD VANNKRAFT PR. 01. 01. 1990 NR V 32 VASSDRAGSDIREKTORATET Forside: Rørgate til Rekvatn krafstasjon,Nordland (foto:T. Jensen) zdzi NVE NORGES VASSDRAGS- OG ENERGIVERK TITTEL NR UTBYGDVANNKRAFTPR.01.01.1990 V 32 FORFATTER(E)/SAKSBEHANDLER(E) DATO September 1990 Torodd Jensen, Erik Kielland, Anders Korvald, Susan Norbom, Jan Slapgård ISBN 82-410-0095-2 ISSN 0802-0191 SAMMENDRAG Publikasjonen inneholder oppgaver over utbygd vannkraft i Norge referert til 533 kraftverk over 1 MW. Disse har en samlet midlere årlig produksjon på 107 816 GWh og maksimal ytelse på 26 557 MW. ABSTRACT This publication contains a statistical survey of developed hydro- power in Norway. There are 533 plants over 1 MW in operation as of 1 January 1990, with a total mean annual generation of 107 816 GWh and maximum capacity of 26 557 MW. EMNEORD SUBJECT TERMS Vannkraft Hydropower ANSVARLIG UNDERSKRIFT Pål Mellqu Vassdragsdi ektør Telefon: (02) 95 95 95 Bankgiro: 0629.05.75026 Kontoradresse: Middelthunsgate 29 Telex: 79 397 NVE0 N Postadresse: Postboks 5091. Maj. Telefax: (02) 95 90 00 Postgiro: 5 05 20 55 0301 Oslo 3 011 Andvord á UTBYGD VANNKRAFT PR. 01.01.1990 INNHOLD SIDE Contents Page FORORD 2 Forward UTBYGD VANNKRAFT 4 Developedhydropower FYLKESOVERSIKT 8 Listofplantsbycounty Østfold 10 Akershus 11 Oslo 12 Hedmark 13 Oppland 14 Buskerud 16 Vestfold 18 Telemark 19 Aust-Agder 21 Vest-Agder 22 Rogaland 24 Hordaland 26 Sogn og Fjordane 30 Møre og Romsdal 34 Sør-Trøndelag 37 Nord - Trøndelag 39 Nordland 41 Troms 45 Finnmark 47 BILAG: FYLKESKART OVER UTBYGD VANNKRAFT Appendix:Countymaps 2 1. -

Norwegian Hydrological Reference Dataset for Climate Change Studies
Norwegian Hydrological Reference Dataset for Climate Change Studies Anne K. Fleig (Ed.) 2 2013 RAPPORT Norwegian Hydrological Reference Dataset for Climate Change Studies Norwegian Water Resources and Energy Directorate 2013 Report no. 2 – 2013 Norwegian Hydrological Reference Dataset for Climate Change Studies Published by: Norwegian Water Resources and Energy Directorate Editor: Anne K. Fleig Authors: Anne K. Fleig, Liss M. Andreassen, Emma Barfod, Jonatan Haga, Lars Egil Haugen, Hege Hisdal, Kjetil Melvold, Tuomo Saloranta Print: Norwegian Water Resources and Energy Directorate Number printed: 50 Femundsenden, spring 2000, Photo: Vidar Raubakken and Cover photo: Gunnar Haugen, NVE. ISSN: 1501-2832 ISBN: 978-82-410-0869-6 Abstract: Based on the Norwegian hydrological measurement network, NVE has selected a Hydrological Reference Dataset for studies of hydrological change. The dataset meets international standards with high data quality. It is suitable for monitoring and studying the effects of climate change on the hydrosphere and cryosphere in Norway. The dataset includes streamflow, groundwater, snow, glacier mass balance and length change, lake ice and water temperature in rivers and lakes. Key words: Reference data, hydrology, climate change Norwegian Water Resources and Energy Directorate Middelthunsgate 29 P.O. Box 5091 Majorstua N 0301 OSLO NORWAY Telephone: +47 22 95 95 95 Fax: +47 22 95 90 00 E-mail: [email protected] Internet: www.nve.no January 2013 Contents Preface ................................................................................................ -

Samer I Østerdalen? En Studie Av Etnisitet I Jernalderen
Samer i Østerdalen? En studie av etnisitet i jernalderen og middelalderen i det nordøstre Hedmark Jostein Bergstøl Universitetet i Oslo 2008 Innholdsfortegnelse: Kapittel 1.................................................................................................................................... 1 1.1. Innledning........................................................................................................................ 1 1.1.1. Overordnede problemstillinger ................................................................................ 1 1.1.2. Presentasjon av studieområdet ................................................................................. 2 1.1.3. Noen begrepsavklaringer.......................................................................................... 3 1.2. Forskningshistorie .......................................................................................................... 3 1.2.1. ”Norrønt” og ”samisk” i forhistorien ....................................................................... 3 1.2.2. Samisk (for)historie.................................................................................................. 5 1.2.3. ”Den Norske Jernalderen”........................................................................................ 6 1.2.4. Fangstfolk i sør.........................................................................................................9 1.2.5. Jernalderen i Østerdalsområdet .............................................................................. 11 -

St.Prp. Nr. 75 (2003–2004)
Utenriksdepartementet St.prp. nr. 75 (2003–2004) Supplering av Verneplan for vassdrag Innhold Del I Den generelle del . 7 8.3 Den samlede Verneplan for vassdrag . 28 1 Innledning og sammendrag . 9 9 De enkelte objekter . 31 2 Verneplan for vassdrag . 10 9.1 Hedmark . 31 9.1.1 Imsa . 31 3 Bakgrunnen for 9.1.2 Sølna . 32 verneplansuppleringen 9.1.3 Tunna . 32 og organiseringen av arbeidet . 11 9.2 Oppland. 33 3.1 Bakgrunnen for 9.2.1 Vismunda . 33 verneplansuppleringen . 11 9.2.2 Vassdragene i Øvre Otta . 34 3.2 Organiseringen av arbeidet. 11 9.2.2.1 Tora . 34 9.2.2.2 Glitra . 34 4 Utvelgelsen av vassdrag for 9.2.2.3 Mosagrovi . 35 vurdering . 12 9.2.2.4 Måråi . 35 4.1 Grunnlaget for utvelgelsen av 9.2.2.5 Åfåtgrovi . 35 vassdrag for vurdering. 12 9.2.3 Jora . 36 4.2 Innkomne forslag til vurdering 9.2.4 Vinda . 37 for vern . 12 9.3 Buskerud . 38 4.3 Forslagene fra styringsgruppen 9.3.1 Nedalselva . 38 og NVE . 12 9.3.2 Dagali (Godfarfoss) . 39 4.4 Departementshøringen . 13 9.4 Vestfold . 40 9.4.1 Dalelva . 40 5 Forholdet til Samlet plan og 9.5 Telemark. 41 nasjonale laksevassdrag . 18 9.5.1 Kåla . 41 5.1 Forholdet til Samlet plan . 18 9.5.2 Digeråi . 41 5.2 Forholdet til andre runde av 9.5.3 Rauda . 42 nasjonale laksevassdrag . 18 9.5.4 Skoevassdraget . 43 9.6 Aust-Agder . 44 6 Verneplanens virkninger. 20 9.6.1 Tovdalsvassdraget ovenfor 6.1 Verneplanens rettslige status . -

Ekkoloddregistreringer I Storsjøen I Rendalen, Dsensjøen Og Engeren, Sommeren Og Høsten 1985
EKKOLODDREGISTRERINGER I STORSJØEN I RENDALEN, DSENSJØEN OG ENGEREN, SOMMEREN OG HØSTEN 1985. Rapport nr. 6 - 1986. av Arne LinlØkken & Tor Qvenild Forord De store sjøene i Hedmark utgjør ca. 45 % av det totale ferskvannsarealet i fylket. De pelagiske fiskebestandene vil være dominerende. Det vil derfor være av avgjØrende betydning å få kartlagt disse ressursenes stØrrelse og bestandssammensetning som grunnlag for en bedre forvaltning. Det er derfor gjort en del forsØk med hydroakustisk utstyr (ekkolodd) som er utviklet av Torfinn Lindem i Direktoratet for naturforvaltning (DN). Til undersØkelsene i 1985 er det benyttet et ekkolodd som velvilligst er utlånt av fiskekontoret i ON. Hamar 6/6 - 86 1 INNLEDNING Bruk av hydroakustisk utstyr til mengde-bestemmelse av fisk i ferskvann er blitt vanlig etter at det har blitt utviklet lett utstyr som er lett å montere og kan brukes i vanlige småbåter (Lindem 1981, Bagenal et al. 1982, Lindem & Sandlund 1984). Metoden er særlig egnet i større innsjØer med relativt stor pelagial-sone og med pelagiale fiskebestander som sik, lagesild og røye. Registreringene bØr kombineres med prØvefiske for å bestemme artssammensetningen og for å relatere ekkoloddata til reelle fiskestØrreiser. Lindem & Sandlund (1984) har gitt regresjonslikningen for sammenhengen mellom mål styrke (TS, i -dB) og logaritmen til fiske lengden (L, i cm) for trål- og flytegarnfangster av krØkle, lagesild og sik i MjØsa: TS = 19.72 x 10gL - 68.08 Likningen brukes også i andre pelagiske fiskebestander og antas å gi et godt bilde av lengdefordelingen utifra ekkoloddsignalene. Sommeren og hØsten 1985 ble det av miljØvernavdelingen i Hedmark fore tatt ekkolodd-registreringer i storsjøen i Rendalen, Osensjøen, Engeren, SØlensjØen, AtnsjØen og Savalen. -
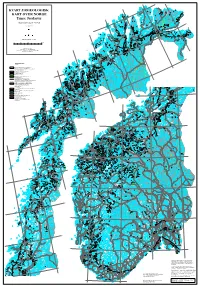
Jordarter V E U N O T N a Leirpollen
30°E 71°N 28°E Austhavet Berlevåg Bearalváhki 26°E Mehamn Nordkinnhalvøya KVARTÆRGEOLOGISK Båtsfjord Vardø D T a e n a Kjøllefjord a n f u j o v r u d o e Oksevatnet t n n KART OVER NORGE a Store L a Buevatnet k Geatnjajávri L s Varangerhalvøya á e Várnjárga f g j e o 24°E Honningsvåg r s d Tema: Jordarter v e u n o t n a Leirpollen Deanodat Vestertana Quaternary map of Norway Havøysund 70°N en rd 3. opplag 2013 fjo r D e a T g tn e n o a ra u a a v n V at t j j a n r á u V Porsanger- Vadsø Vestre Kjæsvatnet Jakobselv halvøya o n Keaisajávri Geassájávri o Store 71°N u Bordejávrrit v Måsvatn n n i e g Havvannet d n r evsbotn R a o j s f r r Kjø- o Bugøy- e fjorden g P fjorden 22°E n a Garsjøen Suolo- s r Kirkenes jávri o Mohkkejávri P Sandøy- Hammerfest Hesseng fjorden Rypefjord t Bjørnevatn e d n Målestokk (Scale) 1:1 mill. u Repparfjorden s y ø r ø S 0 25 50 100 Km Sørøya Sør-Varanger Sállan Skáiddejávri Store Porsanger Sametti Hasvik Leaktojávri Kartet inngår også i B áhèeveai- NASJONALATLAS FOR NORGE 20°E Leavdnja johka u a Lopphavet -

Vannkraftressursenehedmark1994
Vannkraftressursene i Hedmark Tittel Dato Vannkraftressursenei Hedmark - utnyttelse / vern September 1994 Forfatter ISBN 82-7555-043-2 Are Mobæk Sammendrag Rapporten beskriver utnyttelsen av vannkraftressursene i Hedmark fylke pr. 1994. Av et samlet potensiale på 6.3 TWh er 2.3 TWh utnyttet i eksisterende kraftverk. 2.7 TWh er vernet mot kraftutbygging. mens 1.3 TWh gjenstår som restpotensiale for en eventuell framtidig utnytting. Hovedinnholdet i rapporten består a\' korte faktabeskrivelser av enkeltobjekter innenfor de ulike gruppene - utbygd vannkraft. Samlet Plan-prosjekter og vernevassdrag. Abstract The report gives a brief review of the utilization of the hydro power resources in Hedmark county in Norway. Out of a total potensial of 6.3 TW. 2.3 TWh has already been developed. 2.7 TWh has been formally protected against hydro power development while 1.3 TWh remains as a resource for future uti- lization. The maincontents of the report is however a collection of individual descriptions of existing hydro power plants. remaining potentials discribed in the Norwegian Master Plan for Water Resourcesand descriptions of protected watercources. Emneord / Subject terms Ansvarlig underskrift Vannkraftressurser - utbygd. restpo- tensiale. vern I Hydro power resources - utilized, remaining potentials. protected water- Aree Mobækl, cources. Vassdragsforvalter Fvlkesmannen i Hedmark Miljoverndepartementet Miljovernavdelingen Postboks 8013 -dep Fylkeshuset 0030 Oslo 2300 Hamar Norges vassdrags- og energiverk Energiforsyningens Fellesorgansiasjon Postboks 5091 -Majorstua Postboks 274 0301 Oslo 1324 Lsaker Vannkraftressursene i Hedmark Forord for langt storre brukergrupper. Rapporten er utarbeidet av vassdrags- Denne rapporten er utarbeidet for a gi forvalter Are Mobæk ved fylkesman- en samlet. systematisk oversikt over nem miljøvern-avdeling i Hedmark.Det utnyttelsen av vannkraftressursene i har i tilknytning til prosjektet vert ned- Hedmark fylke. -

Stedsnavn I Nord-Østerdal
Stedsnavn i Nord-Østerdal JON OLAV RYEN Stedsnavn i Nord-Østerdal med Rondane, Rørosfjella og Femundstraktene KOLOFON FORLAG © Jon Olav Ryen Stedsnavn i Nord-Østerdal med Rondane, Rørosfjella og Femundstraktene Kolofon Forlag AS 2015 Forside- og baksidefoto: Rondane, med Midtronden og Digerronden (foto: Geir Olav Slåen). Prosjektet produseres på oppdrag fra forfatter/oppdragsgiver/utgiver Jon Olav Ryen Alle rettigheter/ansvar for prosjektets innhold tillegges Jon Olav Ryen Henvendelser utover bestilling av produktet bes rettet til Jon Olav Ryen ISBN 978-82-300-1287-1 Produksjon: Kolofon Forlag AS, 2015 Boken kan kjøpes i bokhandelen eller www.kolofon.com Det må ikke kopieres fra denne boken i strid med Åndsverkloven eller avtaler om kopiering inngått med KOPINOR, interesseorgan for rettighetshavere til åndsverk. Kopiering i strid med lov eller avtale medfører erstatningsansvar og inndraging og kan straffes med bøter eller fengsel. Innhold Forord ..................................................... 7 Innledning ............................................. 8 Bakgrunn, formål og metode ................. 8 Utvalg av stedsnavn ............................... 10 Skrivemåte og oppsett ............................ 12 Henvisninger, bruk av kilder ................ 13 Lydskrift .................................................. 14 Forkortelser, forklaringer ...................... 15 Stedsnavn .............................................. 17 Sørsamiske terrengord ....................... 483 Kilder .................................................... -

Samordning Av Lokaliteter Og Framtidige Utfordringer
Statlig program for forurensingsovervåking Nasjonale programmer for innsjøovervåking Samordning av lokaliteter og framtidige utfordringer TA-1949/2003 ISBN 82-577-4320-8 Referer til denne rapporten som: SFT, 2003. Nasjonale programmer for innsjøovervåking - Samordning av lokaliteter og framtidige utfordringer. (TA-1949/2003) Oppdragsgivere: Statens forurensningstilsyn Postboks 8100 Dep. 0032 Oslo Utførende institusjoner: Norsk institutt for naturforskning Tungasletta 2 7485 Trondheim Norsk institutt for vannforskning Postboks 173 Kjelsås 0411 Oslo Akvaplan-niva AS 9296 Tromsø Forord SFT har i e-mail 24.06.2002 anmodet NIVA om å koordinere et prosjektforslag i samarbeid med Akvaplan-niva og NINA angående fremtidig overvåking av innsjøer og koordinering av ulike overvåkingsprogrammer. Det er et ønske fra SFT om å samordne utvalget av innsjølokaliteter i seks nasjonale overvåkingsprogrammer og se dette i lys av framtidige planer for overvåking og i forbindelse med implementering av Vanndirektivet. Målsetningen er å skaffe en bedre oversikt over de aktivitetene som har foregått til nå, for om mulig å redusere kostnader ved fremtidig feltarbeid og innsamling. Videre ønsker SFT å dra nytte av informasjonen fra forskjellige overvåkingsprogrammer for å få større kunnskap om tilstanden i hver enkelt innsjø. Denne oversikten skal også være utgangspunkt for utvalg av lokaliteter til framtidig overvåking. Denne rapporten gir en oversikt over lokaliteter og aktiviteter i ca. 1000 lokaliteter i 6 nasjonale overvåkingsprogrammer og diskuterer mulighetene -

Join the Worldwide HI Community Bli En Del Av HI-Familien!
About us Om oss Hostelling International Norway Hostelling International Norge • Non-profit membership organisation. • Ideell medlemsorganisasjon. • Proud history since 1932 and a relevant philosophy. • En stolt vandrerhjemshistorie siden 1932. Hostels in • Hostelling International is one of the world’s largest • Hostelling International er en av verdens største Norway 2018 youth membership organisations medlemsorganisasjoner for unge ay - 3.4 million members - 3,4 millioner medlemmer BecomeNorw a member of - Choice of 3,900 youth hostels worldwide, all of which - Et nettverk av 3,900 hosteller over hele verden, som meet internationally assured quality standards. BecomeHI Norwaya member of alle møter internasjonale standarder. • Much more than just a place to stay – we are working - Mye mer enn bare en seng – vi jobber for bedre Join us, getHI discounts Norway and support our towards better understanding between people by forståelse mellom mennesker gjennom å se nye steder, Becomenon-profit organisationa member that brings of discovering new places, new cultures and making oppleve nye kulturer og få venner for livet. Join us, getyoungHI discounts peopleNorway together! and support our lifelong friendships. non-profit organisation that brings • Vårt formål er å skape sosiale møteplasser. Dette gjør www.hihostels.no • Our mission is to create social meeting places by Join us, get discounts and support our vi ved å tilby rimelig overnatting for enkeltreisende, young people together! providing affordable accommodation for individuals, non-profit organisation that brings familier, grupper og organisasjoner. www.hihostels.no families, groups and organisations. young people together! • Hos oss er alle velkomne, uansett kulturell bakgrunn, • Everyone is welcome, regardless of cultural back- www.hihostels.no hudfarge eller religiøs tilhørighet. -

Towards Environmental Design in Hydropower Reservoirs Developing a Handbook for Mitigation Measures in Regulated Lakes
10 Towards environmental design in nr. hydropower reservoirs Developing a handbook for mitigation measures in regulated lakes Ingeborg P. Helland, Stein I. Johnsen, Antti P. Eloranta HydroCen The main objective of HydroCen (Norwegian Research Centre for Hydropower Technology) is to enable the Norwegian hydropower sector to meet complex challenges and exploit new opportunities through innovative technological solutions. The research areas include: • Hydropower structures • Turbine and generators • Market and services • Environmental design The Norwegian University of Science and Technology (NTNU) is the host institution and is the main research partner together with SINTEF Energy Research and the Norwegian Institute for Nature Research (NINA). HydroCen has about 50 national and international partners from industry, R&D institutes and universities. HydroCen is a Centre for Environment-friendly Energy Research (FME). The FME scheme is established by the Norwegian Research Council. The objective of the Research Council of Norway FME-scheme is to establish time-limited research centres, which conduct concentrated, focused and long-term research of high international calibre in order to solve specific challenges in the field. The FME-centres can be established for a maximum period of eight years HydroCen was established in 2016. Towards environmental design in hydropower reservoirs Developing a handbook for mitigation measures in regulated lakes Ingeborg P. Helland Stein I. Johnsen A n tt i P. Eloranta HydroCen Report 10 Helland, I.P., Johnsen, S.I., Eloranta, A.P. 2019. Towards environmental design in hydropower reservoirs - Developing a handbook for mitigation measures in regulated lakes. HydroCen Report 10. Norwegian Research Centre for Hydropower Technology, 59 p. Trondheim, May 2019 ISSN: 2535-5392 (digital publication Pdf) ISBN: 978-82-93602-11-8 Ingeborg P. -

Geology of the Folldal Area, Southern Trondheirr Region Caledonides, Norway
Geology of the Folldal area, southern Trondheirr Region Caledonides, Norway TERJE BJERKGA RD & ARNE BJ0RLYKK E Bjerkqard ,T. & Bjor lykke. A. 1994: Geology of the Folldal area, southern Trondheim Region Caledonides, Norway. Nor. geol. unders. Bull. 426, 53-75. The lowest stratigraphic unit in the Folldal area is the Gula Group, com prising turbidites and minor greenstones in the lower part (the Sinqsas Formation) and graphitic pelites with subordinate marble and conglome rate in the upper part (the Asli Formation). The overly ing Funds]o Group is bimodal with basalts and rhyodacit es, com agma tic gabbros and a large suovotcanlc trondhjem itic intrusion . The bimodality, high content of volcanlclastics, interca lations of continental sediments, geochemistry and lead isotopes of massive sulphides reveal that the Fundsje Gro up represents an island arc acc umulation formed close to a continent. The contact between the Fund sje and Gula Gro ups is interpreted to be primary, as shown by a grad ual lithological transition and no break in metamor phism and structural elements across the contact. The Folldal Trondhjemite in the Fundsjo Group has yielded a U Pb zircon age of 488 ± 2 Ma. Relationships between published ages from the Gula Group and rocks in the Seve Nappes suggest a possible tectonic and metamorphic link between the Seve and the lower part of the Gula Group . Either a primary unconformity or a thrus t marks the contac t between the Asli and Sinqsas Formations in the Gula Gro up. T. BjerkgaJd & A. Bjartykke I), Department of Geology, University of Oslo, P.o.Box 1047, Blindern, N-0316 Oslo, Norway.