Acanthamoeba Is an Evolutionary Ancestor of Macrophages: a Myth Or Reality? Ruqaiyyah Siddiqui Aga Khan University
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Phagocytosis Dynamics Depends on Target Shape
Biophysical Journal Volume 105 September 2013 1143–1150 1143 Phagocytosis Dynamics Depends on Target Shape Debjani Paul,†‡6* Sarra Achouri,†6 Young-Zoon Yoon,† Jurgen Herre,§ Clare E. Bryant,{ and Pietro Cicuta† † ‡ Cavendish Laboratory, University of Cambridge, Cambridge, United Kingdom; Department of Biosciences and Bioengineering, § { Indian Institute of Technology Bombay, Powai, Mumbai, India; Department of Medicine and Department of Veterinary Medicine, University of Cambridge, Cambridge, United Kingdom ABSTRACT A complete understanding of phagocytosis requires insight into both its biochemical and physical aspects. One of the ways to explore the physical mechanism of phagocytosis is to probe whether and how the target properties (e.g., size, shape, surface states, stiffness, etc.) affect their uptake. Here we report an imaging-based method to explore phagocytosis kinetics, which is compatible with real-time imaging and can be used to validate existing reports using fixed and stained cells. We measure single-event engulfment time from a large number of phagocytosis events to compare how size and shape of targets determine their engulfment. The data shows an increase in the average engulfment time for increased target size, for spherical particles. The uptake time data on nonspherical particles confirms that target shape plays a more dominant role than target size for phagocytosis: Ellipsoids with an eccentricity of 0.954 and much smaller surface areas than spheres were taken up five times more slowly than spherical targets. INTRODUCTION Macrophages can engulf a wide variety of targets, such as the degradation of phagosomal contents and the induction invading pathogens, dead cells, foreign airborne particles of the appropriate immune responses. -

Actin Nucleator Spire 1 Is a Regulator of Ectoplasmic Specialization in the Testis Qing Wen1,Nanli1,Xiangxiao 1,2,Wing-Yeelui3, Darren S
Wen et al. Cell Death and Disease (2018) 9:208 DOI 10.1038/s41419-017-0201-6 Cell Death & Disease ARTICLE Open Access Actin nucleator Spire 1 is a regulator of ectoplasmic specialization in the testis Qing Wen1,NanLi1,XiangXiao 1,2,Wing-yeeLui3, Darren S. Chu1, Chris K. C. Wong4, Qingquan Lian5,RenshanGe5, Will M. Lee3, Bruno Silvestrini6 and C. Yan Cheng 1 Abstract Germ cell differentiation during the epithelial cycle of spermatogenesis is accompanied by extensive remodeling at the Sertoli cell–cell and Sertoli cell–spermatid interface to accommodate the transport of preleptotene spermatocytes and developing spermatids across the blood–testis barrier (BTB) and the adluminal compartment of the seminiferous epithelium, respectively. The unique cell junction in the testis is the actin-rich ectoplasmic specialization (ES) designated basal ES at the Sertoli cell–cell interface, and the apical ES at the Sertoli–spermatid interface. Since ES dynamics (i.e., disassembly, reassembly and stabilization) are supported by actin microfilaments, which rapidly converts between their bundled and unbundled/branched configuration to confer plasticity to the ES, it is logical to speculate that actin nucleation proteins play a crucial role to ES dynamics. Herein, we reported findings that Spire 1, an actin nucleator known to polymerize actins into long stretches of linear microfilaments in cells, is an important regulator of ES dynamics. Its knockdown by RNAi in Sertoli cells cultured in vitro was found to impede the Sertoli cell tight junction (TJ)-permeability barrier through changes in the organization of F-actin across Sertoli cell cytosol. Unexpectedly, Spire 1 knockdown also perturbed microtubule (MT) organization in Sertoli cells cultured in vitro. -

Chloroplast Incorporation and Long-Term Photosynthetic Performance Through the Life Cycle in Laboratory Cultures of Elysia Timid
Chloroplast incorporation and long-term photosynthetic performance through the life cycle in laboratory cultures of Elysia timida (Sacoglossa, Heterobranchia) Schmitt et al. Schmitt et al. Frontiers in Zoology 2014, 11:5 http://www.frontiersinzoology.com/content/11/1/5 Schmitt et al. Frontiers in Zoology 2014, 11:5 http://www.frontiersinzoology.com/content/11/1/5 RESEARCH Open Access Chloroplast incorporation and long-term photosynthetic performance through the life cycle in laboratory cultures of Elysia timida (Sacoglossa, Heterobranchia) Valerie Schmitt1,2, Katharina Händeler2, Susanne Gunkel2, Marie-Line Escande3, Diedrik Menzel4, Sven B Gould2, William F Martin2 and Heike Wägele1* Abstract Introduction: The Mediterranean sacoglossan Elysia timida is one of the few sea slug species with the ability to sequester chloroplasts from its food algae and to subsequently store them in a functional state in the digestive gland cells for more than a month, during which time the plastids retain high photosynthetic activity (= long-term retention). Adult E. timida have been described to feed on the unicellular alga Acetabularia acetabulum in their natural environment. The suitability of E. timida as a laboratory model culture system including its food source was studied. Results: In contrast to the literature reporting that juvenile E. timida feed on Cladophora dalmatica first, and later on switch to the adult diet A. acetabulum, the juveniles in this study fed directly on A. acetabulum (young, non-calcified stalks); they did not feed on the various Cladophora spp. (collected from the sea or laboratory culture) offered. This could possibly hint to cryptic speciation with no clear morphological differences, but incipient ecological differentiation. -

Cell & Molecular Biology
BSC ZO- 102 B. Sc. I YEAR CELL & MOLECULAR BIOLOGY DEPARTMENT OF ZOOLOGY SCHOOL OF SCIENCES UTTARAKHAND OPEN UNIVERSITY BSCZO-102 Cell and Molecular Biology DEPARTMENT OF ZOOLOGY SCHOOL OF SCIENCES UTTARAKHAND OPEN UNIVERSITY Phone No. 05946-261122, 261123 Toll free No. 18001804025 Fax No. 05946-264232, E. mail [email protected] htpp://uou.ac.in Board of Studies and Programme Coordinator Board of Studies Prof. B.D.Joshi Prof. H.C.S.Bisht Retd.Prof. Department of Zoology Department of Zoology DSB Campus, Kumaun University, Gurukul Kangri, University Nainital Haridwar Prof. H.C.Tiwari Dr.N.N.Pandey Retd. Prof. & Principal Senior Scientist, Department of Zoology, Directorate of Coldwater Fisheries MB Govt.PG College (ICAR) Haldwani Nainital. Bhimtal (Nainital). Dr. Shyam S.Kunjwal Department of Zoology School of Sciences, Uttarakhand Open University Programme Coordinator Dr. Shyam S.Kunjwal Department of Zoology School of Sciences, Uttarakhand Open University Haldwani, Nainital Unit writing and Editing Editor Writer Dr.(Ms) Meenu Vats Dr.Mamtesh Kumari , Professor & Head Associate. Professor Department of Zoology, Department of Zoology DAV College,Sector-10 Govt. PG College Chandigarh-160011 Uttarkashi (Uttarakhand) Dr.Sunil Bhandari Asstt. Professor. Department of Zoology BGR Campus Pauri, HNB (Central University) Garhwal. Course Title and Code : Cell and Molecular Biology (BSCZO 102) ISBN : 978-93-85740-54-1 Copyright : Uttarakhand Open University Edition : 2017 Published By : Uttarakhand Open University, Haldwani, Nainital- 263139 Contents Course 1: Cell and Molecular Biology Course code: BSCZO102 Credit: 3 Unit Block and Unit title Page number Number Block 1 Cell Biology or Cytology 1-128 1 Cell Type : History and origin. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -
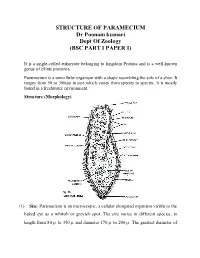
STRUCTURE of PARAMECIUM Dr Poonam Kumari Dept of Zoology (BSC PART I PAPER I)
STRUCTURE OF PARAMECIUM Dr Poonam kumari Dept Of Zoology (BSC PART I PAPER I) It is a single-celled eukaryote belonging to kingdom Protista and is a well-known genus of ciliate protozoa. Paramecium is a unicellular organism with a shape resembling the sole of a shoe. It ranges from 50 to 300um in size which varies from species to species. It is mostly found in a freshwater environment. Structure (Morphology) (1) Size: Paramecium is an microscopic, a cellular elongated organism visible to the baked eye as a whitish or greyish spot. The size varies in different species, in length from 80 to 350 and diameter 170 to 290 . The greatest diameter of the cylindrical body is about two third of its entire length. Usually the individuals of the same species may show minor morphological and physiological differences. (2) Shape: Paramecium is a slipper shaped, cigar shaped, or spindle shaped animalcule. Its shape is usually constant and a symmetrical, because slipper like shape. The body is elongated, blunt and rounded at the anterior end and somewhat pointed of the posterior end. In cross section it is circular with greatest diameter behind the centre of body. The anterior half of the body is slightly twisted. The body is distinguished into an oral or ventral surface and an aboral or dorsal surface. The structure is more complicated due to the development of certain organelles in the acellular body. (3) Oral groove: The ventral surface of body bears a prominent, oblique and shallow depression is called oral groove, it arise from the middle of body and extends to the left side of anterior end. -

EE Just's "Independent Irritability"
ESSAY Molecular Reproduction & Development 76:966–974 (2009) E.E. Just’s ‘‘Independent Irritability’’ Revisited: The Activated Egg as Excitable Soft Matter STUART A. NEWMAN* Department of Cell Biology and Anatomy, New York Medical College, Valhalla, New York SUMMARY Ernest Everett Just’s experimental work on post-fertilization events in invertebrate eggs led him to posit a dynamic and directive role for the zygotic ‘‘ectoplasm’’ (cortical Just was correct in his estimation cytoplasm), in subsequent development. His perspective was neglected during the of the ‘‘informational’’ role of the years that followed his early death not only because of his well-documented margina- ectoplasm’s dynamics. lization as an African-American in U.S. science, but because his ideas were at odds with the growing gene-centrism of developmental biology in the latter half of the 20th century. This essay reviews experimental work that shows that the egg cortex in many animal groups is a chemically and mechanically active medium that sustains both spatiotemporal calcium ion transients and periodic deformations in the time leading up * Corresponding author: to cleavage. These wave phenomena are seen to play regulatory roles in germ plasm Department of Cell Biology and localization and gene expression, and influence the reliability and success of devel- Anatomy opmental outcomes. Just resisted vitalistic explanations for the active processes he New York Medical College Basic Science Building observed and inferred regarding the egg cortical cytoplasm, but recognized that the Valhalla, NY 10595. physics and chemistry of his time were inadequate to account for these phenomena E-mail: [email protected] and anticipated that expansions of these fields would be necessary to explain them. -

Cytoskeleton and Cell Motility
Cytoskeleton and Cell Motility 1. (28 pts) Amoeba proteus, a single-celled eukaryote, moves by means of psudopods attaching to and detaching from the substratum. Locomotion seems to be correlated with the forward flow of fluid cytoplasm (endoplasm) into an advancing pseudopod through a surrounding, gel-like ectoplasmic tube. The ectoplasm forms at the pseudopodial tip in a region called the Fountain Zone. As the amoeba advances the ectoplasmic tube “liquifies” at the posterior end to form endoplasm. These features are illustrated in the figure below. Both locomotion and cytoplasmic streaming are inhibited by cytochalasin B. Consider everything you’ve learned so far and answer all the following questions. A. (4 pts) When amoeba undergoes cell division, it stops streaming and rounds up into a spherical cell. Describe how this change in shape and behavior comes about and why it might be a necessary precondition for division. B. (6 pts) Briefly describe how cytoplasmic streaming is most likely organized and generated at the cellular and molecular levels. C. (5 pts) Briefly describe an additional experiment or observation that would test your hypothesis and indicate clearly what the results would show. CCM - 1 Cytoskeleton and Cell Motility D. (8 pts) Describe clearly, with the aid of a well-labeled diagram, how streaming within a pseudopod could result in movement of the amoeba across the substratum. E. (5 pts) Describe how your streaming mechanism might be regulated such that the amoeba might change its streaming pattern to form phagocytic pseudopods around a ciliate it had touched. Now evaluate some past answers to these questions, in light of your own essays. -

Structure and Development of the Egg of the Glossiphoniid Leech Theromyzon Rude: Characterization of Developmental Stages and Structure of the Early Uncleaved Egg
Development 100, 211-225 (1987) 211 Printed in Great Britain © The Company of Biologists Limited 1987 Structure and development of the egg of the glossiphoniid leech Theromyzon rude: characterization of developmental stages and structure of the early uncleaved egg JUAN FERNANDEZ, NANCY OLEA and CECILIA MATTE Departamento de Biologia, Facultad de Ciendas, Untversidad de Chile, Casilla 653, Santiago, Chile Summary Some aspects of the reproductive biology of the meridional bands, during stage le, lead to accumu- glossiphoniid leech, Theromyzon rude, under labora- lation of ooplasm at both egg poles. In this manner, tory conditions, and the staging and structure of its the teloplasm or pole plasm forms. Completion of the uncleaved egg were studied. Sexually mature animals first cleavage furrow, by the end of stage If, is form breeding communities and fertilization occurs in preceded by dorsoventral flattening of the egg and the ovLsacs, presumably around the time of egg rearrangement of its teloplasm and perinuclear laying. Opposition may be postponed for hours or plasm. Structure of the early uncleaved egg has been days, but the eggs in the ovisacs remain blocked at studied with transmission and scanning electron mi- first meiotic metaphase. Development of the croscopy of intact or permeabilized preparations. The uncleaved egg, from the time of oviposit ion to com- plasmalemma forms numerous long and some short pletion of the first cleavage division, has been sub- microvilli evenly distributed across the egg surface. divided into six stages. At 20 °C, the six developmental The ectoplasm includes many vesicles, mitochondria, stages take 5-6 h. Characterization of' the stages is granules and an elaborate network of filament based on observations of both live and fixed/cleared bundles. -

Protists: Amoeba
Microorganisms – Protists: Amoeba The amoeba is a protozoan that belongs to the Kingdom Protista. The name amoeba comes from the Greek word amoibe, which means change. (Amoeba is also spelled ameba.) Protists are microscopic unicellular organisms that don't fit into the other kingdoms. Some protozoans are considered plant- like while others are considered animal-like. The amoeba is considered an animal-like protist because it moves and consumes its food. Protists are classified by how they move, some have cilia or flagella, but the ameba has an unusual way of creeping along by stretching its cytoplasm into fingerlike extensions called pseudopodia. The word "pseudopodia" means "false foot". Label the pseudopodia. When looking at ameba under a microscope, an observer will note that no amoeba looks the same as any other. The cell membrane is very flexible and allows for the amoeba to change shape. Amoebas live in ponds or puddles, and some can even live inside people. Color and label the cell membrane red. There are two types of cytoplasm in the amoeba. The darker cytoplasm toward the interior of the protozoan is called endoplasm. The clearer cytoplasm that is found near the cell membrane is called ectoplasm. On the diagram, the endoplasm is indicated by the dotted area, and the ectoplasm by the white area. Color and label the endoplasm pink. Color and label the ectoplasm light blue. By pushing the endoplasm toward the cell membrane, the amoeba causes its body to extend and creep along. It is also by this method that the amoeba consumes its food. -

Difference Between Ectoplasm and Endoplasm Key Difference – Ectoplasm Vs Endoplasm
Difference Between Ectoplasm and Endoplasm www.differencebetween.com Key Difference – Ectoplasm vs Endoplasm Protozoa are single-cell eukaryotic organisms. They resemble animal cells and contain major organelles and the cell nucleus. Protozoan’s cytoplasm has two distinct areas called ectoplasm and endoplasm. The outer layer of the cytoplasm is known as the ectoplasm. The inner layer is known as the endoplasm. The terms endoplasm and ectoplasm are used mainly to describe amoeba cytoplasm and how it helps feeding and locomotion. Amoeba is a single cell eukaryotic organism which is made up of a nucleus and cytoplasm. The cytoplasm of amoeba can be divided into the two layers: endoplasm and ectoplasm. Ectoplasm is the clear outer cytoplasmic layer of amoeba while endoplasm is the inner granule-rich cytoplasmic layer of amoeba. This is the key difference between ectoplasm and endoplasm. What is Ectoplasm? Ectoplasm refers to the outer layer of the cytoplasm of a cell. It is not a granulated area. This part of the cytoplasm is watery and clear. Ectoplasm is located immediately adjacent to the plasma membrane. It is clearly visible in the amoeba cell. Figure 01: Cell Structure Amoeba Amoeba cells locomote by pseudopodia formation. The ectoplasm of the amoeba cell is responsible for changing the direction of the pseudopodium. Location of the pseudopodium changes when the alkalinity and acidity of the water in ectoplasm are changed. A slight change in the acidity or alkalinity is enough for the flowing of cytoplasm which helps in locomotion. Water concentration of the amoeba cell is regulated by the endoplasm. Endoplasm easily absorbs or releases water through the partially permeable membrane.