Studies on the Organization and Localization of Actin and Myosin in Neurons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acanthamoeba Is an Evolutionary Ancestor of Macrophages: a Myth Or Reality? Ruqaiyyah Siddiqui Aga Khan University
eCommons@AKU Department of Biological & Biomedical Sciences Medical College, Pakistan February 2012 Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality? Ruqaiyyah Siddiqui Aga Khan University Naveed Ahmed Khan Aga Khan University Follow this and additional works at: http://ecommons.aku.edu/pakistan_fhs_mc_bbs Part of the Biochemistry Commons Recommended Citation Siddiqui, R., Khan, N. (2012). Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality?. Experimental Parasitology, 130(2), 95-97. Available at: http://ecommons.aku.edu/pakistan_fhs_mc_bbs/16 Experimental Parasitology 130 (2012) 95–97 Contents lists available at SciVerse ScienceDirect Experimental Parasitology journal homepage: www.elsevier.com/locate/yexpr Minireview Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality? ⇑ Ruqaiyyah Siddiqui a, Naveed Ahmed Khan a,b, a Aga Khan University, Stadium Road, Karachi, Pakistan b School of Veterinary Medicine and Science, University of Nottingham, Sutton Bonington, England, UK article info abstract Article history: Given the remarkable similarities in cellular structure (morphological and ultra-structural features), Received 26 October 2011 molecular motility, biochemical physiology, ability to capture prey by phagocytosis and interactions with Received in revised form 17 November 2011 microbial pathogens, here we pose the question whether Acanthamoeba and macrophages are evolution- Accepted 19 November 2011 ary related. This is discussed in the light of evolution and functional aspects such as the astonishing Available online 28 November 2011 resemblance of many bacteria to infect and multiply inside human macrophages and amoebae in analo- gous ways. Further debate and studies will determine if Acanthamoeba is an evolutionary ancestor of Keywords: macrophages. Is this a myth or reality? Acanthamoeba Ó 2011 Elsevier Inc. -
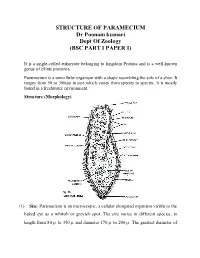
STRUCTURE of PARAMECIUM Dr Poonam Kumari Dept of Zoology (BSC PART I PAPER I)
STRUCTURE OF PARAMECIUM Dr Poonam kumari Dept Of Zoology (BSC PART I PAPER I) It is a single-celled eukaryote belonging to kingdom Protista and is a well-known genus of ciliate protozoa. Paramecium is a unicellular organism with a shape resembling the sole of a shoe. It ranges from 50 to 300um in size which varies from species to species. It is mostly found in a freshwater environment. Structure (Morphology) (1) Size: Paramecium is an microscopic, a cellular elongated organism visible to the baked eye as a whitish or greyish spot. The size varies in different species, in length from 80 to 350 and diameter 170 to 290 . The greatest diameter of the cylindrical body is about two third of its entire length. Usually the individuals of the same species may show minor morphological and physiological differences. (2) Shape: Paramecium is a slipper shaped, cigar shaped, or spindle shaped animalcule. Its shape is usually constant and a symmetrical, because slipper like shape. The body is elongated, blunt and rounded at the anterior end and somewhat pointed of the posterior end. In cross section it is circular with greatest diameter behind the centre of body. The anterior half of the body is slightly twisted. The body is distinguished into an oral or ventral surface and an aboral or dorsal surface. The structure is more complicated due to the development of certain organelles in the acellular body. (3) Oral groove: The ventral surface of body bears a prominent, oblique and shallow depression is called oral groove, it arise from the middle of body and extends to the left side of anterior end. -

EE Just's "Independent Irritability"
ESSAY Molecular Reproduction & Development 76:966–974 (2009) E.E. Just’s ‘‘Independent Irritability’’ Revisited: The Activated Egg as Excitable Soft Matter STUART A. NEWMAN* Department of Cell Biology and Anatomy, New York Medical College, Valhalla, New York SUMMARY Ernest Everett Just’s experimental work on post-fertilization events in invertebrate eggs led him to posit a dynamic and directive role for the zygotic ‘‘ectoplasm’’ (cortical Just was correct in his estimation cytoplasm), in subsequent development. His perspective was neglected during the of the ‘‘informational’’ role of the years that followed his early death not only because of his well-documented margina- ectoplasm’s dynamics. lization as an African-American in U.S. science, but because his ideas were at odds with the growing gene-centrism of developmental biology in the latter half of the 20th century. This essay reviews experimental work that shows that the egg cortex in many animal groups is a chemically and mechanically active medium that sustains both spatiotemporal calcium ion transients and periodic deformations in the time leading up * Corresponding author: to cleavage. These wave phenomena are seen to play regulatory roles in germ plasm Department of Cell Biology and localization and gene expression, and influence the reliability and success of devel- Anatomy opmental outcomes. Just resisted vitalistic explanations for the active processes he New York Medical College Basic Science Building observed and inferred regarding the egg cortical cytoplasm, but recognized that the Valhalla, NY 10595. physics and chemistry of his time were inadequate to account for these phenomena E-mail: [email protected] and anticipated that expansions of these fields would be necessary to explain them. -

Cytoskeleton and Cell Motility
Cytoskeleton and Cell Motility 1. (28 pts) Amoeba proteus, a single-celled eukaryote, moves by means of psudopods attaching to and detaching from the substratum. Locomotion seems to be correlated with the forward flow of fluid cytoplasm (endoplasm) into an advancing pseudopod through a surrounding, gel-like ectoplasmic tube. The ectoplasm forms at the pseudopodial tip in a region called the Fountain Zone. As the amoeba advances the ectoplasmic tube “liquifies” at the posterior end to form endoplasm. These features are illustrated in the figure below. Both locomotion and cytoplasmic streaming are inhibited by cytochalasin B. Consider everything you’ve learned so far and answer all the following questions. A. (4 pts) When amoeba undergoes cell division, it stops streaming and rounds up into a spherical cell. Describe how this change in shape and behavior comes about and why it might be a necessary precondition for division. B. (6 pts) Briefly describe how cytoplasmic streaming is most likely organized and generated at the cellular and molecular levels. C. (5 pts) Briefly describe an additional experiment or observation that would test your hypothesis and indicate clearly what the results would show. CCM - 1 Cytoskeleton and Cell Motility D. (8 pts) Describe clearly, with the aid of a well-labeled diagram, how streaming within a pseudopod could result in movement of the amoeba across the substratum. E. (5 pts) Describe how your streaming mechanism might be regulated such that the amoeba might change its streaming pattern to form phagocytic pseudopods around a ciliate it had touched. Now evaluate some past answers to these questions, in light of your own essays. -

Structure and Development of the Egg of the Glossiphoniid Leech Theromyzon Rude: Characterization of Developmental Stages and Structure of the Early Uncleaved Egg
Development 100, 211-225 (1987) 211 Printed in Great Britain © The Company of Biologists Limited 1987 Structure and development of the egg of the glossiphoniid leech Theromyzon rude: characterization of developmental stages and structure of the early uncleaved egg JUAN FERNANDEZ, NANCY OLEA and CECILIA MATTE Departamento de Biologia, Facultad de Ciendas, Untversidad de Chile, Casilla 653, Santiago, Chile Summary Some aspects of the reproductive biology of the meridional bands, during stage le, lead to accumu- glossiphoniid leech, Theromyzon rude, under labora- lation of ooplasm at both egg poles. In this manner, tory conditions, and the staging and structure of its the teloplasm or pole plasm forms. Completion of the uncleaved egg were studied. Sexually mature animals first cleavage furrow, by the end of stage If, is form breeding communities and fertilization occurs in preceded by dorsoventral flattening of the egg and the ovLsacs, presumably around the time of egg rearrangement of its teloplasm and perinuclear laying. Opposition may be postponed for hours or plasm. Structure of the early uncleaved egg has been days, but the eggs in the ovisacs remain blocked at studied with transmission and scanning electron mi- first meiotic metaphase. Development of the croscopy of intact or permeabilized preparations. The uncleaved egg, from the time of oviposit ion to com- plasmalemma forms numerous long and some short pletion of the first cleavage division, has been sub- microvilli evenly distributed across the egg surface. divided into six stages. At 20 °C, the six developmental The ectoplasm includes many vesicles, mitochondria, stages take 5-6 h. Characterization of' the stages is granules and an elaborate network of filament based on observations of both live and fixed/cleared bundles. -

Protists: Amoeba
Microorganisms – Protists: Amoeba The amoeba is a protozoan that belongs to the Kingdom Protista. The name amoeba comes from the Greek word amoibe, which means change. (Amoeba is also spelled ameba.) Protists are microscopic unicellular organisms that don't fit into the other kingdoms. Some protozoans are considered plant- like while others are considered animal-like. The amoeba is considered an animal-like protist because it moves and consumes its food. Protists are classified by how they move, some have cilia or flagella, but the ameba has an unusual way of creeping along by stretching its cytoplasm into fingerlike extensions called pseudopodia. The word "pseudopodia" means "false foot". Label the pseudopodia. When looking at ameba under a microscope, an observer will note that no amoeba looks the same as any other. The cell membrane is very flexible and allows for the amoeba to change shape. Amoebas live in ponds or puddles, and some can even live inside people. Color and label the cell membrane red. There are two types of cytoplasm in the amoeba. The darker cytoplasm toward the interior of the protozoan is called endoplasm. The clearer cytoplasm that is found near the cell membrane is called ectoplasm. On the diagram, the endoplasm is indicated by the dotted area, and the ectoplasm by the white area. Color and label the endoplasm pink. Color and label the ectoplasm light blue. By pushing the endoplasm toward the cell membrane, the amoeba causes its body to extend and creep along. It is also by this method that the amoeba consumes its food. -

Difference Between Ectoplasm and Endoplasm Key Difference – Ectoplasm Vs Endoplasm
Difference Between Ectoplasm and Endoplasm www.differencebetween.com Key Difference – Ectoplasm vs Endoplasm Protozoa are single-cell eukaryotic organisms. They resemble animal cells and contain major organelles and the cell nucleus. Protozoan’s cytoplasm has two distinct areas called ectoplasm and endoplasm. The outer layer of the cytoplasm is known as the ectoplasm. The inner layer is known as the endoplasm. The terms endoplasm and ectoplasm are used mainly to describe amoeba cytoplasm and how it helps feeding and locomotion. Amoeba is a single cell eukaryotic organism which is made up of a nucleus and cytoplasm. The cytoplasm of amoeba can be divided into the two layers: endoplasm and ectoplasm. Ectoplasm is the clear outer cytoplasmic layer of amoeba while endoplasm is the inner granule-rich cytoplasmic layer of amoeba. This is the key difference between ectoplasm and endoplasm. What is Ectoplasm? Ectoplasm refers to the outer layer of the cytoplasm of a cell. It is not a granulated area. This part of the cytoplasm is watery and clear. Ectoplasm is located immediately adjacent to the plasma membrane. It is clearly visible in the amoeba cell. Figure 01: Cell Structure Amoeba Amoeba cells locomote by pseudopodia formation. The ectoplasm of the amoeba cell is responsible for changing the direction of the pseudopodium. Location of the pseudopodium changes when the alkalinity and acidity of the water in ectoplasm are changed. A slight change in the acidity or alkalinity is enough for the flowing of cytoplasm which helps in locomotion. Water concentration of the amoeba cell is regulated by the endoplasm. Endoplasm easily absorbs or releases water through the partially permeable membrane. -

Chondrogenesis, Studied with the Electron Microscope
CHONDROGENESIS, STUDIED WITH THE ELECTRON MICROSCOPE GABRIEL C. GODMAN, M.1)., and KEITH R. PORTER, Ph.I). From The Rockefeller Institute. Dr. Godman's present address is Department of Microbiology, Columbia University, New York ABSTRACT The role of the cells in the fabrication of a connective tissue matrix, and the structural modifications which accompany cytodifferentiation have been investigated in developing epiphyseal cartilage of fetal rat by means of electron microscopy. Differentiation of the pre- chondral mesenchymal cells to chondroblasts is marked by the acquisition of an extensive endoplasmic reticulum, enlargement and concentration of the Golgi apparatus, the ap- pearance of membrane-bounded cytoplasmic inclusions, and the formation of specialized foci of increased density in the cell cortex. These modifications are related to the secretion of the cartilage matrix. The matrix of young hyaline cartilage consists of groups of rela- tively short, straight, banded collagen fibrils of 10 to 20 m# and a dense granular component embedded in an amorphous ground substance of moderate electron density. It is postu- lated that the first phase of fibrillogenesis takes place at the cell cortex in dense bands or striae within the ectoplasm subjacent to the cell membrane. These can be resolved into sheaves of "primary" fibrils of about 7 to 10 m#. They are supposedly shed (by excortica- tion) into the matrix space between the separating chondroblasts, where they may serve as "cores" of the definitive matrix fibrils. The diameter of the fibrils may subsequently increase up to threefold, presumably by incorporation of "soluble" or tropocollagen units from the ground substance. The chondroblast also discharges into the matrix the electron- dense amorphous or granular contents of vesicles derived from the Golgi apparatus, and the mixed contents of large vacuoles or blebs bounded by distinctive double membranes. -

35 Cell Locomotion
The Dynamic Cell 34 Locomotion S.K. Maciver Jan '03 Cell Locomotion Cells move for a variety of reasons. Bacteria swim by beating their flagella in order to exploit newly created micro- environments and amoeba crawl to gather bacteria to feed on. The cells of vertebrates must move to heal wounds, fend off invaders and the eukaryotic flagella is used to propel sperm cells toward eggs. Cell type Speed (mm/sec) Scale speed* Bacteria 10 10x length sec Ciliate 1,000 100 Amoeba proteus 3 0.006 Neutrophil 0.1 0.01 Fibroblast 0.01 0.0002 Table 3 Speed record of different types of cell locomotion. *Scale speed expressed as the body lengths that the organism covers every second (not really serious). The Prokaryotic Flagellum Bacteria invented the wheel! The bacterial flagellum is a helical structure that drives the cell through the media like a propeller. Hook flagellin The structure is rigid and turned by a rotatory motor at the base Universal joint where it connects to the bacteria's body. The rotary motor consist of several wheel-like discs one of which the M-ring (and/or L-ring possibly the S-ring) interact with the C-ring and studs to rotate the P-ring whole structure. The rotary motor is very like a stepping motor! The flagella is composed of a protein called flagellin which is synthesized in the cell body and transported through the narrow lumen of the growing flagella itself to polymerise at the tip as it is about to exit the bacteria! This system has evolved into a syringe – like mechanism to inject toxins into the cells of vertebrates during infection (this is called "type 3 secretion"). -

A Comparative Electron Microscopic Study on Ehrlich Ascites Cancerous
A Comparative Electron Microscopic Study on Ehrlich Ascites Tumor Cells, Yoshida Sarcoma Cells, and Human Cancerous Peritonitis Ascites Cells G. YASUZUMIANDR. SUGIHARA (Laboratory for Electron Microscope Research, Department of Anatomy, Nara Medical College,'Nara Pref., Japan) It is known that the Ehrlich ascites tumor gave no growth. An operation was performed is of epithelial origin, while the Yoshida sarcoma at the hospital, revealing a tumor the size of originates from the reticuloendothelium (13). The a hen-egg in the pyloric region. The pancreas present paper deals with a comparative electron and retroperitoneal regions showed diffuse meta- microscopic study of uninfected Ehrlich mouse static inflammation which made gastrectomy im ascites tumor (EAT) cells, Yoshida sarcoma (YS) possible. cells, and human cancerous peritonitis ascites Approximately 0.5 ml. of ascites fluid was re (HCPA) cells. A morphological analysis of the moved by capillary pipette from the peritoneal EAT by electron microscopy had already revealed cavity and placed immediately in 1 per cent virus-like particles in the ground substance of osmium tetroxide buffered at pH 7.3 with veronal- the cytoplasm (5, 6, 8, 9, 12). Recent progress acetate. After fixation for 30 minutes the cells achieved via the electron microscope in the study were directly dehydrated, without being washed of the submicroscopic structure of the uninfected in distilled water, in a series of increasing concen EAT cells renewed interest in the virus-like par trations of ethyl alcohol and embedded in a mix ticles associated with the endoplasmic reticulum ture of methyl methacrylate and n-butyl methac- (1, 3, 11). Although Wessel and Bernhard (8) rylate. -

Mechanics and Control of the Cytoskeleton in Amoeba Proteus
Mechanics and control of the cytoskeleton in Amoeba proteus Micah Dembo Theoretical Biology and Biophysics, Theoretical Division, Los Alamos National Laboratory, Los Alamos, New Mexico 87545 ABSTRACT Many models of the cyto- accounts for the kinematics of the Several dynamical factors are crucial skeletal motility of Amoeba proteus can cytoskeleton: the detailed velocity field to the success of the minimal model be formulated in terms of the theory of of the forward flow of the endoplasm, and are likely to be general features of reactive interpenetrating flow (Dembo the contraction of the ectoplasmic cytoskeletal mechanics and control in and Harlow, 1986). We have devised tube, and the inversion of the flow in the amoeboid cells. These are: a constitu- numerical methodology for testing such fountain zone. The model also gives a tive law for the viscosity of the contrac- models against the phenomenon of satisfactory account of measurements tile network that includes an automatic steady axisymmetric fountain flow. The of pressure gradients, measurements process of gelation as the network simplest workable scheme revealed by of heat dissipation, and measurements density gets large; a very vigorous such tests (the minimal model) is the of the output of useful work by amoe- cycle of network polymerization and main preoccupation of this study. All ba. Finally, the model suggests a very depolymerization (in the case of A. parameters of the minimal model are promising (but still hypothetical) con- proteus, the time constant for this reac- determined from available data. Using tinuum formulation of the free boundary tion is z12 s); control of network con- these parameters the model quantita- problem of amoeboid motion. -

Cytoplasts Made from Human Blood Polymorphonuclear Leukocytes with Or Without Heat
Proc. Natl. Acad. Sci. USA Vol. 84, pp. 454-458, January 1987 Cell Biology Cytoplasts made from human blood polymorphonuclear leukocytes with or without heat: Preservation of both motile function and respiratory burst oxidase activity (cytokineplasts/chemotaxis/phagocytosis/superoxide) STEPHEN E. MALAWISTA AND GRETCHEN VAN BLARICOM Department of Internal Medicine, Yale University School of Medicine, New Haven, CT 06510 Communicated by Aaron B. Lerner, October 1, 1986 ABSTRACT Anucleate fragments (cytoplasts) from poly- MATERIALS AND METHODS morphonuclear leukocytes (PMN) are simplified systems that Preparation of Human Blood Leukocytes. As described (8), can be used to elucidate specific pathways by which cell heparinized venous blood (250-300 ml) from normal volun- function is altered. PMN cytoplasts in current use are defective teers was sedimented in dextran, and the leukocyte-rich either in activatable respiratory burst oxidase activity or in supernatant (60-85% PMN) was sedimented and washed in motile function. By centrifugation of PMN on discontinuous modified heparinized Krebs-Ringer phosphate buffer (pH gradients of Ficoll without cytochalasin B, we have created 7.4). The cells were osmotically shocked (to lyse erythro- granule-poor cytoplasts in which both these capacities are cytes), restored to isotonicity, washed once more in buffer, preserved. Specifically, they generate superoxide anion (°2 ) and counted (yield, generally S x 108 to 1 x 109 PMN). and reduce nitroblue tetrazolium dye on appropriate stimula- Aliquots (5 x 107 to 1 x 108) of cells to be heated were tion; they respond chemotactically to erythrocytes destroyed by sedimented (5 sec) in 1.7-ml plastic microcentrifuge tubes laser microirradiation or to the specific chemoattractants (Eppendorf centrifuge 5412, Brinkmann), the supernatants fMet-Leu-Phe (10 nM) and C5a (zymosan-activated serum); were removed, the tubes were plunged into a water bath at and they ingest and kill staphylococci.