From Channels to Cross Bridges
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

James Spudich, Ph.D. the Myosin Mesa and Beyond: on the Underlying Molecular Basis of Hyper-Contractility Caused by Hypertrophic Card
JAMES SPUDICH, PH.D. THE MYOSIN MESA AND BEYOND: ON THE UNDERLYING MOLECULAR BASIS OF HYPER-CONTRACTILITY CAUSED BY HYPERTROPHIC CARD APRIL 6, 2017 3:00 P.M. 208 LIGHT HALL SPONSORED BY: THE DEPARTMENT OF CELL AND DEVELOPMENTAL BIOLOGY Upcoming Discovery Lecture: ERIC GOUAUX, PH.D. Senior Scientist, Vollum Institute Jennifer and Bernard Lacroute Term Chair in Neuroscience Research Investigator, HHMI April 20, 2017 208 Light Hall / 4:00 P.M. 1190-4347-Institution-Discovery Lecture Series-Spudich-BK-CH.indd 1 3/21/17 11:11 AM THE MYOSIN MESA AND BEYOND: ON THE UNDERLYING MOLECULAR BASIS OF HYPER-CONTRACTILITY CAUSED BY JAMES SPUDICH, PH.D. STANFORD UNIVERSITY SCHOOL OF MEDICINE DEPARTMENT OF BIOCHEMISTRY THE MYOSIN MESA AND BEYOND: DOUGLASS M. AND NOLA LEISHMAN PROFESSOR OF ON THE UNDERLYING MOLECULAR BASIS CARDIOVASCULAR DISEASE OF HYPER-CONTRACTILITY CAUSED BY MEMBER, NATIONAL ACADEMY OF SCIENCES HYPERTROPHIC CARD After 40 years of developing and utilizing assays to understand the James Spudich, Douglass M. and Nola Leishman Professor of molecular basis of energy transduction by the myosin family of Cardiovascular Disease, is in the Department of Biochemistry at Stanford molecular motors, all members of my laboratory are now focused on University School of Medicine. He received his B.S. in chemistry from understanding the underlying biochemical and biophysical bases of the University of Illinois in 1963 and his Ph.D. in biochemistry from human hypertrophic (HCM) and dilated (DCM) cardiomyopathies. Stanford in 1968. He did postdoctoral work in genetics at Stanford and Our primary focus is on HCM since these mutations cause the heart in structural biology at the MRC Laboratory in Cambridge, England. -

By Krista Conger It Was Around 3 P.M. on a Thursday Last October When James Spudich and Suzanne Pfeffer Poked Their Heads Into T
BECKMAN CENTER FOR MOLECULAR AND GENETIC MEDICINE PG. 6 BECKMAN RESEARCHERS MAP INTRICACIES OF LUNG CANCER IN ONE OF THEIR OWN By Krista Conger Spudich had no way of knowing it, but the meeting he’d interrupted between Krasnow and assistant professor of pediatrics Christin Kuo, MD, had been It was around 3 p.m. on a Thursday last October when called to discuss how Krasnow could broaden his James Spudich and Suzanne Pfeffer poked their heads research, which he had been conducting mainly in into the office of Mark Krasnow, on the fourth floor of mice and small primates called mouse lemurs, to the Beckman Center for Molecular and Genetic include human cancers. But the logistical and legal Medicine, interrupting a meeting he was having with hoops that would need to be cleared prior to any a colleague. kind of human-based research were daunting, and Krasnow knew it would likely take months to obtain “Can we talk to you for a minute?” Pfeffer said. the necessary approvals. Impromptu conversations were nothing new for the Kuo, a specialist in pulmonary medicine, had been three. The longtime colleagues and friends have a lot to working with Stephen Quake, PhD, professor of talk about. Spudich, PhD, whose research focuses on bioengineering and of applied physics and co-president understanding the molecular forces that drive muscle of the Chan Zuckerberg Biohub, and postdoctoral contractions, earned a doctoral degree from Stanford scholar Spyros Darmanis, PhD, a group leader at the in 1968 under Arthur Kornberg, MD, a founding Biohub, to study the development and function of lung member of the Department of Biochemistry. -

Acanthamoeba Is an Evolutionary Ancestor of Macrophages: a Myth Or Reality? Ruqaiyyah Siddiqui Aga Khan University
eCommons@AKU Department of Biological & Biomedical Sciences Medical College, Pakistan February 2012 Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality? Ruqaiyyah Siddiqui Aga Khan University Naveed Ahmed Khan Aga Khan University Follow this and additional works at: http://ecommons.aku.edu/pakistan_fhs_mc_bbs Part of the Biochemistry Commons Recommended Citation Siddiqui, R., Khan, N. (2012). Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality?. Experimental Parasitology, 130(2), 95-97. Available at: http://ecommons.aku.edu/pakistan_fhs_mc_bbs/16 Experimental Parasitology 130 (2012) 95–97 Contents lists available at SciVerse ScienceDirect Experimental Parasitology journal homepage: www.elsevier.com/locate/yexpr Minireview Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality? ⇑ Ruqaiyyah Siddiqui a, Naveed Ahmed Khan a,b, a Aga Khan University, Stadium Road, Karachi, Pakistan b School of Veterinary Medicine and Science, University of Nottingham, Sutton Bonington, England, UK article info abstract Article history: Given the remarkable similarities in cellular structure (morphological and ultra-structural features), Received 26 October 2011 molecular motility, biochemical physiology, ability to capture prey by phagocytosis and interactions with Received in revised form 17 November 2011 microbial pathogens, here we pose the question whether Acanthamoeba and macrophages are evolution- Accepted 19 November 2011 ary related. This is discussed in the light of evolution and functional aspects such as the astonishing Available online 28 November 2011 resemblance of many bacteria to infect and multiply inside human macrophages and amoebae in analo- gous ways. Further debate and studies will determine if Acanthamoeba is an evolutionary ancestor of Keywords: macrophages. Is this a myth or reality? Acanthamoeba Ó 2011 Elsevier Inc. -
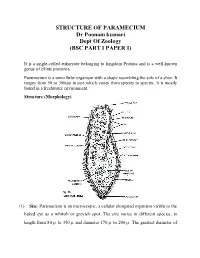
STRUCTURE of PARAMECIUM Dr Poonam Kumari Dept of Zoology (BSC PART I PAPER I)
STRUCTURE OF PARAMECIUM Dr Poonam kumari Dept Of Zoology (BSC PART I PAPER I) It is a single-celled eukaryote belonging to kingdom Protista and is a well-known genus of ciliate protozoa. Paramecium is a unicellular organism with a shape resembling the sole of a shoe. It ranges from 50 to 300um in size which varies from species to species. It is mostly found in a freshwater environment. Structure (Morphology) (1) Size: Paramecium is an microscopic, a cellular elongated organism visible to the baked eye as a whitish or greyish spot. The size varies in different species, in length from 80 to 350 and diameter 170 to 290 . The greatest diameter of the cylindrical body is about two third of its entire length. Usually the individuals of the same species may show minor morphological and physiological differences. (2) Shape: Paramecium is a slipper shaped, cigar shaped, or spindle shaped animalcule. Its shape is usually constant and a symmetrical, because slipper like shape. The body is elongated, blunt and rounded at the anterior end and somewhat pointed of the posterior end. In cross section it is circular with greatest diameter behind the centre of body. The anterior half of the body is slightly twisted. The body is distinguished into an oral or ventral surface and an aboral or dorsal surface. The structure is more complicated due to the development of certain organelles in the acellular body. (3) Oral groove: The ventral surface of body bears a prominent, oblique and shallow depression is called oral groove, it arise from the middle of body and extends to the left side of anterior end. -

EE Just's "Independent Irritability"
ESSAY Molecular Reproduction & Development 76:966–974 (2009) E.E. Just’s ‘‘Independent Irritability’’ Revisited: The Activated Egg as Excitable Soft Matter STUART A. NEWMAN* Department of Cell Biology and Anatomy, New York Medical College, Valhalla, New York SUMMARY Ernest Everett Just’s experimental work on post-fertilization events in invertebrate eggs led him to posit a dynamic and directive role for the zygotic ‘‘ectoplasm’’ (cortical Just was correct in his estimation cytoplasm), in subsequent development. His perspective was neglected during the of the ‘‘informational’’ role of the years that followed his early death not only because of his well-documented margina- ectoplasm’s dynamics. lization as an African-American in U.S. science, but because his ideas were at odds with the growing gene-centrism of developmental biology in the latter half of the 20th century. This essay reviews experimental work that shows that the egg cortex in many animal groups is a chemically and mechanically active medium that sustains both spatiotemporal calcium ion transients and periodic deformations in the time leading up * Corresponding author: to cleavage. These wave phenomena are seen to play regulatory roles in germ plasm Department of Cell Biology and localization and gene expression, and influence the reliability and success of devel- Anatomy opmental outcomes. Just resisted vitalistic explanations for the active processes he New York Medical College Basic Science Building observed and inferred regarding the egg cortical cytoplasm, but recognized that the Valhalla, NY 10595. physics and chemistry of his time were inadequate to account for these phenomena E-mail: [email protected] and anticipated that expansions of these fields would be necessary to explain them. -

Cytoskeleton and Cell Motility
Cytoskeleton and Cell Motility 1. (28 pts) Amoeba proteus, a single-celled eukaryote, moves by means of psudopods attaching to and detaching from the substratum. Locomotion seems to be correlated with the forward flow of fluid cytoplasm (endoplasm) into an advancing pseudopod through a surrounding, gel-like ectoplasmic tube. The ectoplasm forms at the pseudopodial tip in a region called the Fountain Zone. As the amoeba advances the ectoplasmic tube “liquifies” at the posterior end to form endoplasm. These features are illustrated in the figure below. Both locomotion and cytoplasmic streaming are inhibited by cytochalasin B. Consider everything you’ve learned so far and answer all the following questions. A. (4 pts) When amoeba undergoes cell division, it stops streaming and rounds up into a spherical cell. Describe how this change in shape and behavior comes about and why it might be a necessary precondition for division. B. (6 pts) Briefly describe how cytoplasmic streaming is most likely organized and generated at the cellular and molecular levels. C. (5 pts) Briefly describe an additional experiment or observation that would test your hypothesis and indicate clearly what the results would show. CCM - 1 Cytoskeleton and Cell Motility D. (8 pts) Describe clearly, with the aid of a well-labeled diagram, how streaming within a pseudopod could result in movement of the amoeba across the substratum. E. (5 pts) Describe how your streaming mechanism might be regulated such that the amoeba might change its streaming pattern to form phagocytic pseudopods around a ciliate it had touched. Now evaluate some past answers to these questions, in light of your own essays. -

Structure and Development of the Egg of the Glossiphoniid Leech Theromyzon Rude: Characterization of Developmental Stages and Structure of the Early Uncleaved Egg
Development 100, 211-225 (1987) 211 Printed in Great Britain © The Company of Biologists Limited 1987 Structure and development of the egg of the glossiphoniid leech Theromyzon rude: characterization of developmental stages and structure of the early uncleaved egg JUAN FERNANDEZ, NANCY OLEA and CECILIA MATTE Departamento de Biologia, Facultad de Ciendas, Untversidad de Chile, Casilla 653, Santiago, Chile Summary Some aspects of the reproductive biology of the meridional bands, during stage le, lead to accumu- glossiphoniid leech, Theromyzon rude, under labora- lation of ooplasm at both egg poles. In this manner, tory conditions, and the staging and structure of its the teloplasm or pole plasm forms. Completion of the uncleaved egg were studied. Sexually mature animals first cleavage furrow, by the end of stage If, is form breeding communities and fertilization occurs in preceded by dorsoventral flattening of the egg and the ovLsacs, presumably around the time of egg rearrangement of its teloplasm and perinuclear laying. Opposition may be postponed for hours or plasm. Structure of the early uncleaved egg has been days, but the eggs in the ovisacs remain blocked at studied with transmission and scanning electron mi- first meiotic metaphase. Development of the croscopy of intact or permeabilized preparations. The uncleaved egg, from the time of oviposit ion to com- plasmalemma forms numerous long and some short pletion of the first cleavage division, has been sub- microvilli evenly distributed across the egg surface. divided into six stages. At 20 °C, the six developmental The ectoplasm includes many vesicles, mitochondria, stages take 5-6 h. Characterization of' the stages is granules and an elaborate network of filament based on observations of both live and fixed/cleared bundles. -

Curriculum Vitae James A. Spudich, Ph.D
Curriculum Vitae James A. Spudich, Ph.D. Douglass M. and Nola Leishman Professor of Cardiovascular Disease Department of Biochemistry Stanford University School of Medicine EDUCATION 1963 University of Illinois, Chemistry Dept of Biochemistry B.S. 1968 Stanford University, Biochemistry Dept of Biochemistry Ph.D. 1968-1969 Stanford University, Molecular Genetics Dept of Biological Sciences Postdoctoral 1969-1971 Cambridge University, Structural Biology MRC Lab of Mol Biol Postdoctoral PROFESSIONAL EXPERIENCE 2012 Co-Founder, MyoKardia, Inc. 2011-present Adjunct Professor, Institute of Stem Cell Biology and Regenerative Medicine (inStem), Bangalore, India 2005-present Adjunct Professor, National Centre for Biological Sciences (NCBS) of the Tata Institute of Fundamental Research (TIFR), Bangalore, India; and TIFR, Bombay, India 1998-2002 Co-Founder and first Director, Interdisciplinary Program in Bioengineering, Biomedicine and Biosciences – Bio-X, Stanford University 1998 Co-Founder, Cytokinetics, Inc. 1992-present Professor, Dept of Biochemistry, Stanford University School of Medicine (Chairman from 1994-1998) 1989-2011 Professor, Dept of Developmental Biology, Stanford University School of Medicine 1977-1992 Professor, Dept of Structural Biology, Stanford University School of Medicine (Chairman from 1979-1984) 1976-1977 Professor, Dept of Biochemistry and Biophysics, UCSF, San Francisco 1974-1976 Associate Professor, Dept of Biochemistry and Biophysics, UCSF, San Francisco 1971-1974 Assistant Professor, Dept of Biochemistry and Biophysics, -

Protists: Amoeba
Microorganisms – Protists: Amoeba The amoeba is a protozoan that belongs to the Kingdom Protista. The name amoeba comes from the Greek word amoibe, which means change. (Amoeba is also spelled ameba.) Protists are microscopic unicellular organisms that don't fit into the other kingdoms. Some protozoans are considered plant- like while others are considered animal-like. The amoeba is considered an animal-like protist because it moves and consumes its food. Protists are classified by how they move, some have cilia or flagella, but the ameba has an unusual way of creeping along by stretching its cytoplasm into fingerlike extensions called pseudopodia. The word "pseudopodia" means "false foot". Label the pseudopodia. When looking at ameba under a microscope, an observer will note that no amoeba looks the same as any other. The cell membrane is very flexible and allows for the amoeba to change shape. Amoebas live in ponds or puddles, and some can even live inside people. Color and label the cell membrane red. There are two types of cytoplasm in the amoeba. The darker cytoplasm toward the interior of the protozoan is called endoplasm. The clearer cytoplasm that is found near the cell membrane is called ectoplasm. On the diagram, the endoplasm is indicated by the dotted area, and the ectoplasm by the white area. Color and label the endoplasm pink. Color and label the ectoplasm light blue. By pushing the endoplasm toward the cell membrane, the amoeba causes its body to extend and creep along. It is also by this method that the amoeba consumes its food. -

Difference Between Ectoplasm and Endoplasm Key Difference – Ectoplasm Vs Endoplasm
Difference Between Ectoplasm and Endoplasm www.differencebetween.com Key Difference – Ectoplasm vs Endoplasm Protozoa are single-cell eukaryotic organisms. They resemble animal cells and contain major organelles and the cell nucleus. Protozoan’s cytoplasm has two distinct areas called ectoplasm and endoplasm. The outer layer of the cytoplasm is known as the ectoplasm. The inner layer is known as the endoplasm. The terms endoplasm and ectoplasm are used mainly to describe amoeba cytoplasm and how it helps feeding and locomotion. Amoeba is a single cell eukaryotic organism which is made up of a nucleus and cytoplasm. The cytoplasm of amoeba can be divided into the two layers: endoplasm and ectoplasm. Ectoplasm is the clear outer cytoplasmic layer of amoeba while endoplasm is the inner granule-rich cytoplasmic layer of amoeba. This is the key difference between ectoplasm and endoplasm. What is Ectoplasm? Ectoplasm refers to the outer layer of the cytoplasm of a cell. It is not a granulated area. This part of the cytoplasm is watery and clear. Ectoplasm is located immediately adjacent to the plasma membrane. It is clearly visible in the amoeba cell. Figure 01: Cell Structure Amoeba Amoeba cells locomote by pseudopodia formation. The ectoplasm of the amoeba cell is responsible for changing the direction of the pseudopodium. Location of the pseudopodium changes when the alkalinity and acidity of the water in ectoplasm are changed. A slight change in the acidity or alkalinity is enough for the flowing of cytoplasm which helps in locomotion. Water concentration of the amoeba cell is regulated by the endoplasm. Endoplasm easily absorbs or releases water through the partially permeable membrane. -

Michael Sheetz, James Spudich, and Ronald Vale Receive the 2012 Albert Lasker Basic Medical Research Award
Molecules in motion: Michael Sheetz, James Spudich, and Ronald Vale receive the 2012 Albert Lasker Basic Medical Research Award Sarah Jackson J Clin Invest. 2012;122(10):3374-3377. https://doi.org/10.1172/JCI66361. News The Albert and Mary Lasker Foundation honors three pioneers in the field of molecular motor proteins, Michael Sheetz, James Spudich, and Ronald Vale (Figure 1), for their contribution to uncovering how these proteins catalyze movement. Sheetz and Spudich developed the first biochemical assay to reconstitute myosin motor activity and showed that myosin and ATP alone were sufficient to direct transport along actin filaments. Fueled by this discovery, Vale and Sheetz examined transport in giant squid axon extracts and discovered a new motor, kinesin, that moves along microtubules. Cumulatively, their work opened the door for understanding how motor proteins drive transport of a host of molecules involved in numerous cellular processes, including cell polarity, cell division, cellular movement, and signal transduction. Muscle movement Much of the early research on cellular movement focused on understanding muscle contraction. Filaments of actin and myosin form a banding pattern in muscle that is readily visible by light microscopy, with a characteristic striated appearance. The functional unit of muscle, termed a sarcomere, repeats at regular intervals along the muscle fibers. Measurements from contracted, resting, and stretched muscle using high-resolution light microscopy supported the notion that contraction was due to the sliding of actin and myosin filaments (1, 2). In 1969, preeminent British scientist Hugh Huxley proposed that crossbridges that connect actin and myosin generate the sliding […] Find the latest version: https://jci.me/66361/pdf News Molecules in motion: Michael Sheetz, James Spudich, and Ronald Vale receive the 2012 Albert Lasker Basic Medical Research Award The Albert and Mary Lasker Founda- James Spudich joined the Huxley labora- motility along actin. -

Studies on the Organization and Localization of Actin and Myosin in Neurons
STUDIES ON THE ORGANIZATION AND LOCALIZATION OF ACTIN AND MYOSIN IN NEURONS EDWARD R. KUCZMARSKI and JOEL L. ROSENBAUM From the Department of Biology, Yale University, New Haven, Connecticut 06520 ABSTRACT The organization of actin in mouse neuroblastoma and chicken dorsal root ganglion (DRG) nerve cells was investigated by means of a variety of electron microscope techniques. Microspikes of neuroblastoma cells contained bundles of 7- to 8-nm actin filaments which originated in the interior of the neurite. In the presence of high concentrations of Mg ++ ion, filaments in these bundles became highly ordered to form paracrystals. Actin filaments, but not bundles, were observed in growth cones of DRG cells. Actin was localized in the cell body, neurites, and microspikes ot~ both DRG and neuroblastoma nerve cells by fluorescein-labeled S1. Myosin was localized primarily in the neurites of chick DRG nerve cells with fluorescein-labeled anti-brain myosin antibody. This antibody also stained stress fibers in fibroblasts and myoblasts but did not stain muscle myofibrils. KEY WORDS neurons microspikes poisons stops the transport and causes a piling up microfilaments myosin localization of material proximal to the block (44). A retro- grade transport has also been described in which Neurons exhibit a variety of motile phenomena, material moves from the axon tip toward the cell one of the most dramatic examples being nerve body (35). Some form of motility may also be outgrowth. In experiments which proved the neu- required for transmitter release at the synapse (4). ron theory, Harrison (28) first described the rapid These examples demonstrate that several nerve movement of nerve cells in tissue culture where functions involve motility.