EE Just's "Independent Irritability"
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Induction of Cortical Granule Exocytosis of Pig Oocytes by Spermatozoa During Meiotic Maturation W
Induction of cortical granule exocytosis of pig oocytes by spermatozoa during meiotic maturation W. H. Wang, M. Hosoe and Y. Shioya Department of Reproduction, National Institute of Animal Industry, Tsukuba Norindanchi, PO Box 5, Ibaraki 305, Japan Pig oocytes were examined to test their ability to undergo cortical granule exocytosis upon penetration by spermatozoa during meiotic maturation. Immature or maturing oocytes (cultured in vitro for 0 h, 26 h and 46 h) were inseminated with ejaculated boar spermatozoa in vitro. Before and after insemination, oocytes were stained with peanut agglutinin labelled with fluorescein isothiocyanate and the cortical granule distributions were examined under the fluorescent microscope and the laser confocal microscope. Before insemination, all the oocytes at the germinal vesicle stage showed a uniform distribution of cortical granules throughout the cortical cytoplasm. The granules migrated centrifugally during maturation and were distributed just beneath the oolemma in the oocytes after germinal vesicle breakdown, forming a monolayer in metaphase I or metaphase II. Cortical granules were still present in all penetrated oocytes at the germinal vesicle stage 18 h after insemination; in contrast, 26% and 84% of the oocytes inseminated at the stages of germinal vesicle breakdown or at metaphase I and II, respectively, completely released their cortical granules. Nuclear activation rates of penetrated oocytes were 0%, 38% and 96% in oocytes cultured for 0 h, 26 h and 46 h, respectively. Of the nuclear-activated oocytes, 67% (oocytes cultured for 26 h) and 88% (oocytes cultured for 46 h) released cortical granules completely. Complete cortical granule exocytosis was not observed in nuclear-inactivated oocytes. -

Acanthamoeba Is an Evolutionary Ancestor of Macrophages: a Myth Or Reality? Ruqaiyyah Siddiqui Aga Khan University
eCommons@AKU Department of Biological & Biomedical Sciences Medical College, Pakistan February 2012 Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality? Ruqaiyyah Siddiqui Aga Khan University Naveed Ahmed Khan Aga Khan University Follow this and additional works at: http://ecommons.aku.edu/pakistan_fhs_mc_bbs Part of the Biochemistry Commons Recommended Citation Siddiqui, R., Khan, N. (2012). Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality?. Experimental Parasitology, 130(2), 95-97. Available at: http://ecommons.aku.edu/pakistan_fhs_mc_bbs/16 Experimental Parasitology 130 (2012) 95–97 Contents lists available at SciVerse ScienceDirect Experimental Parasitology journal homepage: www.elsevier.com/locate/yexpr Minireview Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality? ⇑ Ruqaiyyah Siddiqui a, Naveed Ahmed Khan a,b, a Aga Khan University, Stadium Road, Karachi, Pakistan b School of Veterinary Medicine and Science, University of Nottingham, Sutton Bonington, England, UK article info abstract Article history: Given the remarkable similarities in cellular structure (morphological and ultra-structural features), Received 26 October 2011 molecular motility, biochemical physiology, ability to capture prey by phagocytosis and interactions with Received in revised form 17 November 2011 microbial pathogens, here we pose the question whether Acanthamoeba and macrophages are evolution- Accepted 19 November 2011 ary related. This is discussed in the light of evolution and functional aspects such as the astonishing Available online 28 November 2011 resemblance of many bacteria to infect and multiply inside human macrophages and amoebae in analo- gous ways. Further debate and studies will determine if Acanthamoeba is an evolutionary ancestor of Keywords: macrophages. Is this a myth or reality? Acanthamoeba Ó 2011 Elsevier Inc. -

Cellular and Molecular Aspects of Oocyte
Santella et al. Zoological Letters (2020) 6:5 https://doi.org/10.1186/s40851-020-00157-5 REVIEW Open Access Cellular and molecular aspects of oocyte maturation and fertilization: a perspective from the actin cytoskeleton Luigia Santella1*, Nunzia Limatola1 and Jong Tai Chun2 Abstract Much of the scientific knowledge on oocyte maturation, fertilization, and embryonic development has come from the experiments using gametes of marine organisms that reproduce by external fertilization. In particular, echinoderm eggs have enabled the study of structural and biochemical changes related to meiotic maturation and fertilization owing to the abundant availability of large and transparent oocytes and eggs. Thus, in vitro studies of oocyte maturation and sperm-induced egg activation in starfish are carried out under experimental conditions that resemble those occurring in nature. During the maturation process, immature oocytes of starfish are released from the prophase of the first meiotic division, and acquire the competence to be fertilized through a highly programmed sequence of morphological and physiological changes at the oocyte surface. In addition, the changes in the cortical and nuclear regions are essential for normal and monospermic fertilization. This review summarizes the current state of research on the cortical actin cytoskeleton in mediating structural and physiological changes during oocyte maturation and sperm and egg activation in starfish and sea urchin. The common denominator in these studies with echinoderms is that exquisite rearrangements of the egg cortical actin filaments play pivotal roles in gamete interactions, Ca2+ signaling, exocytosis of cortical granules, and control of monospermic fertilization. In this review, we also compare findings from studies using invertebrate eggs with what is known about the contributions made by the actin cytoskeleton in mammalian eggs. -
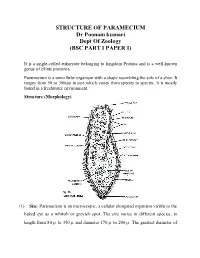
STRUCTURE of PARAMECIUM Dr Poonam Kumari Dept of Zoology (BSC PART I PAPER I)
STRUCTURE OF PARAMECIUM Dr Poonam kumari Dept Of Zoology (BSC PART I PAPER I) It is a single-celled eukaryote belonging to kingdom Protista and is a well-known genus of ciliate protozoa. Paramecium is a unicellular organism with a shape resembling the sole of a shoe. It ranges from 50 to 300um in size which varies from species to species. It is mostly found in a freshwater environment. Structure (Morphology) (1) Size: Paramecium is an microscopic, a cellular elongated organism visible to the baked eye as a whitish or greyish spot. The size varies in different species, in length from 80 to 350 and diameter 170 to 290 . The greatest diameter of the cylindrical body is about two third of its entire length. Usually the individuals of the same species may show minor morphological and physiological differences. (2) Shape: Paramecium is a slipper shaped, cigar shaped, or spindle shaped animalcule. Its shape is usually constant and a symmetrical, because slipper like shape. The body is elongated, blunt and rounded at the anterior end and somewhat pointed of the posterior end. In cross section it is circular with greatest diameter behind the centre of body. The anterior half of the body is slightly twisted. The body is distinguished into an oral or ventral surface and an aboral or dorsal surface. The structure is more complicated due to the development of certain organelles in the acellular body. (3) Oral groove: The ventral surface of body bears a prominent, oblique and shallow depression is called oral groove, it arise from the middle of body and extends to the left side of anterior end. -

Cytoskeleton and Cell Motility
Cytoskeleton and Cell Motility 1. (28 pts) Amoeba proteus, a single-celled eukaryote, moves by means of psudopods attaching to and detaching from the substratum. Locomotion seems to be correlated with the forward flow of fluid cytoplasm (endoplasm) into an advancing pseudopod through a surrounding, gel-like ectoplasmic tube. The ectoplasm forms at the pseudopodial tip in a region called the Fountain Zone. As the amoeba advances the ectoplasmic tube “liquifies” at the posterior end to form endoplasm. These features are illustrated in the figure below. Both locomotion and cytoplasmic streaming are inhibited by cytochalasin B. Consider everything you’ve learned so far and answer all the following questions. A. (4 pts) When amoeba undergoes cell division, it stops streaming and rounds up into a spherical cell. Describe how this change in shape and behavior comes about and why it might be a necessary precondition for division. B. (6 pts) Briefly describe how cytoplasmic streaming is most likely organized and generated at the cellular and molecular levels. C. (5 pts) Briefly describe an additional experiment or observation that would test your hypothesis and indicate clearly what the results would show. CCM - 1 Cytoskeleton and Cell Motility D. (8 pts) Describe clearly, with the aid of a well-labeled diagram, how streaming within a pseudopod could result in movement of the amoeba across the substratum. E. (5 pts) Describe how your streaming mechanism might be regulated such that the amoeba might change its streaming pattern to form phagocytic pseudopods around a ciliate it had touched. Now evaluate some past answers to these questions, in light of your own essays. -

Structure and Development of the Egg of the Glossiphoniid Leech Theromyzon Rude: Characterization of Developmental Stages and Structure of the Early Uncleaved Egg
Development 100, 211-225 (1987) 211 Printed in Great Britain © The Company of Biologists Limited 1987 Structure and development of the egg of the glossiphoniid leech Theromyzon rude: characterization of developmental stages and structure of the early uncleaved egg JUAN FERNANDEZ, NANCY OLEA and CECILIA MATTE Departamento de Biologia, Facultad de Ciendas, Untversidad de Chile, Casilla 653, Santiago, Chile Summary Some aspects of the reproductive biology of the meridional bands, during stage le, lead to accumu- glossiphoniid leech, Theromyzon rude, under labora- lation of ooplasm at both egg poles. In this manner, tory conditions, and the staging and structure of its the teloplasm or pole plasm forms. Completion of the uncleaved egg were studied. Sexually mature animals first cleavage furrow, by the end of stage If, is form breeding communities and fertilization occurs in preceded by dorsoventral flattening of the egg and the ovLsacs, presumably around the time of egg rearrangement of its teloplasm and perinuclear laying. Opposition may be postponed for hours or plasm. Structure of the early uncleaved egg has been days, but the eggs in the ovisacs remain blocked at studied with transmission and scanning electron mi- first meiotic metaphase. Development of the croscopy of intact or permeabilized preparations. The uncleaved egg, from the time of oviposit ion to com- plasmalemma forms numerous long and some short pletion of the first cleavage division, has been sub- microvilli evenly distributed across the egg surface. divided into six stages. At 20 °C, the six developmental The ectoplasm includes many vesicles, mitochondria, stages take 5-6 h. Characterization of' the stages is granules and an elaborate network of filament based on observations of both live and fixed/cleared bundles. -

Two Pathways Regulate Cortical Granule Translocation to Prevent Polyspermy in Mouse Oocytes
ARTICLE Received 12 Jun 2016 | Accepted 27 Oct 2016 | Published 19 Dec 2016 DOI: 10.1038/ncomms13726 OPEN Two pathways regulate cortical granule translocation to prevent polyspermy in mouse oocytes Liam P. Cheeseman1,Je´roˆme Boulanger1, Lisa M. Bond1 & Melina Schuh1,2 An egg must be fertilized by a single sperm only. To prevent polyspermy, the zona pellucida, a structure that surrounds mammalian eggs, becomes impermeable upon fertilization, preventing the entry of further sperm. The structural changes in the zona upon fertilization are driven by the exocytosis of cortical granules. These translocate from the oocyte’s centre to the plasma membrane during meiosis. However, very little is known about the mechanism of cortical granule translocation. Here we investigate cortical granule transport and dynamics in live mammalian oocytes by using Rab27a as a marker. We show that two separate mechanisms drive their transport: myosin Va-dependent movement along actin filaments, and an unexpected vesicle hitchhiking mechanism by which cortical granules bind to Rab11a vesicles powered by myosin Vb. Inhibiting cortical granule translocation severely impaired the block to sperm entry, suggesting that translocation defects could contribute to miscarriages that are caused by polyspermy. 1 Medical Research Council Laboratory of Molecular Biology, Francis Crick Avenue, Cambridge Biomedical Campus, Cambridge CB2 0QH, UK. 2 Max Planck Institute for Biophysical Chemistry, Am Fassberg 11, Go¨ttingen 37077, Germany. Correspondence and requests for materials should be addressed to M.S. (email: [email protected]). NATURE COMMUNICATIONS | 7:13726 | DOI: 10.1038/ncomms13726 | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/ncomms13726 viable human embryo can only develop from an egg that established marker for cortical granules21 (Fig. -

Protists: Amoeba
Microorganisms – Protists: Amoeba The amoeba is a protozoan that belongs to the Kingdom Protista. The name amoeba comes from the Greek word amoibe, which means change. (Amoeba is also spelled ameba.) Protists are microscopic unicellular organisms that don't fit into the other kingdoms. Some protozoans are considered plant- like while others are considered animal-like. The amoeba is considered an animal-like protist because it moves and consumes its food. Protists are classified by how they move, some have cilia or flagella, but the ameba has an unusual way of creeping along by stretching its cytoplasm into fingerlike extensions called pseudopodia. The word "pseudopodia" means "false foot". Label the pseudopodia. When looking at ameba under a microscope, an observer will note that no amoeba looks the same as any other. The cell membrane is very flexible and allows for the amoeba to change shape. Amoebas live in ponds or puddles, and some can even live inside people. Color and label the cell membrane red. There are two types of cytoplasm in the amoeba. The darker cytoplasm toward the interior of the protozoan is called endoplasm. The clearer cytoplasm that is found near the cell membrane is called ectoplasm. On the diagram, the endoplasm is indicated by the dotted area, and the ectoplasm by the white area. Color and label the endoplasm pink. Color and label the ectoplasm light blue. By pushing the endoplasm toward the cell membrane, the amoeba causes its body to extend and creep along. It is also by this method that the amoeba consumes its food. -

Difference Between Ectoplasm and Endoplasm Key Difference – Ectoplasm Vs Endoplasm
Difference Between Ectoplasm and Endoplasm www.differencebetween.com Key Difference – Ectoplasm vs Endoplasm Protozoa are single-cell eukaryotic organisms. They resemble animal cells and contain major organelles and the cell nucleus. Protozoan’s cytoplasm has two distinct areas called ectoplasm and endoplasm. The outer layer of the cytoplasm is known as the ectoplasm. The inner layer is known as the endoplasm. The terms endoplasm and ectoplasm are used mainly to describe amoeba cytoplasm and how it helps feeding and locomotion. Amoeba is a single cell eukaryotic organism which is made up of a nucleus and cytoplasm. The cytoplasm of amoeba can be divided into the two layers: endoplasm and ectoplasm. Ectoplasm is the clear outer cytoplasmic layer of amoeba while endoplasm is the inner granule-rich cytoplasmic layer of amoeba. This is the key difference between ectoplasm and endoplasm. What is Ectoplasm? Ectoplasm refers to the outer layer of the cytoplasm of a cell. It is not a granulated area. This part of the cytoplasm is watery and clear. Ectoplasm is located immediately adjacent to the plasma membrane. It is clearly visible in the amoeba cell. Figure 01: Cell Structure Amoeba Amoeba cells locomote by pseudopodia formation. The ectoplasm of the amoeba cell is responsible for changing the direction of the pseudopodium. Location of the pseudopodium changes when the alkalinity and acidity of the water in ectoplasm are changed. A slight change in the acidity or alkalinity is enough for the flowing of cytoplasm which helps in locomotion. Water concentration of the amoeba cell is regulated by the endoplasm. Endoplasm easily absorbs or releases water through the partially permeable membrane. -

Cortical Granules of the Sea Urchin Translocate Early in Oocyte Maturation
Development 124, 1845-1850 (1997) 1845 Printed in Great Britain © The Company of Biologists Limited 1997 DEV6281 Cortical granules of the sea urchin translocate early in oocyte maturation Linnea K. Berg and Gary M. Wessel* Department of Molecular and Cell Biology and Biochemistry, Brown University, Providence, RI 02912, USA *Author for correspondence (e-mail: [email protected]) SUMMARY Cortical granules are secretory vesicles poised at the cortex ically in cortical granules. We found that the translocation of an egg that, upon stimulation by sperm contact at fer- of cortical granules in in vitro-matured oocytes begins with tilization, secrete their contents. These contents modify the the movement of the germinal vesicle to the oocyte cell extracellular environment and block additional sperm surface, and is 50% complete 1 hour after germinal vesicle from reaching the egg. The role of cortical granules in breakdown. In the in vitro-matured egg, 99% of the blocking polyspermy is conserved throughout much of cortical granules are at the cortex, indistinguishable from phylogeny. In the sea urchin, cortical granules accumulate translocation in oocytes that mature in vivo. We have also throughout the cytoplasm during oogenesis, but in mature found that eggs that mature in vitro are functionally eggs the cortical granules are attached to the plasma identical to eggs that mature in vivo by four criteria. membrane, having translocated to the cortex at some (1) The matured cells undergo a selective turnover of earlier time. To study the process of cortical granule mRNA encoding cortical granule contents. (2) The newly translocation to the cell surface we have devised a formed pronucleus begins transcription of histone procedure for maturation of sea urchin oocytes in vitro. -

Reproductionresearch
REPRODUCTIONRESEARCH Calcium-free vitrification reduces cryoprotectant-induced zona pellucida hardening and increases fertilization rates in mouse oocytes Mark G Larman, Courtney B Sheehan and David K Gardner Colorado Center for Reproductive Medicine, 799 East Hampden Avenue, Suite 520, Englewood, Colorado 80113, USA Correspondence should be addressed to D K Gardner; Email: [email protected] Abstract Despite the success of embryo cyropreservation, routine oocyte freezing has proved elusive with only around 200 children born since the first reported birth in 1986. The reason for the poor efficiency is unclear, but evidence of zona pellucida hardening following oocyte freezing indicates that current protocols affect oocyte physiology. Here we report that two cryoprotectants commonly used in vitrification procedures, dimethyl sulfoxide (DMSO) and ethylene glycol, cause a large transient increase in intracellular calcium concentration in mouse metaphase II (MII) oocytes comparable to the initial increase triggered at fertilization. Removal of extracellular calcium from the medium failed to affect the response exacted by DMSO challenge, but significantly reduced the ethylene glycol-induced calcium increase. These results suggest that the source of the DMSO-induced calcium increase is solely from the internal calcium pool, as opposed to ethylene glycol that causes an influx of calcium across the plasma membrane from the external medium. By carrying out vitrification in calcium-free media, it was found that zona hardening is significantly reduced and subsequent fertilization and development to the two-cell stage significantly increased. Furthermore, such calcium-free treatment appears not to affect the embryo adversely, as shown by development rates to the blastocyst stage and cell number/allocation. -

Studies on the Organization and Localization of Actin and Myosin in Neurons
STUDIES ON THE ORGANIZATION AND LOCALIZATION OF ACTIN AND MYOSIN IN NEURONS EDWARD R. KUCZMARSKI and JOEL L. ROSENBAUM From the Department of Biology, Yale University, New Haven, Connecticut 06520 ABSTRACT The organization of actin in mouse neuroblastoma and chicken dorsal root ganglion (DRG) nerve cells was investigated by means of a variety of electron microscope techniques. Microspikes of neuroblastoma cells contained bundles of 7- to 8-nm actin filaments which originated in the interior of the neurite. In the presence of high concentrations of Mg ++ ion, filaments in these bundles became highly ordered to form paracrystals. Actin filaments, but not bundles, were observed in growth cones of DRG cells. Actin was localized in the cell body, neurites, and microspikes ot~ both DRG and neuroblastoma nerve cells by fluorescein-labeled S1. Myosin was localized primarily in the neurites of chick DRG nerve cells with fluorescein-labeled anti-brain myosin antibody. This antibody also stained stress fibers in fibroblasts and myoblasts but did not stain muscle myofibrils. KEY WORDS neurons microspikes poisons stops the transport and causes a piling up microfilaments myosin localization of material proximal to the block (44). A retro- grade transport has also been described in which Neurons exhibit a variety of motile phenomena, material moves from the axon tip toward the cell one of the most dramatic examples being nerve body (35). Some form of motility may also be outgrowth. In experiments which proved the neu- required for transmitter release at the synapse (4). ron theory, Harrison (28) first described the rapid These examples demonstrate that several nerve movement of nerve cells in tissue culture where functions involve motility.