Mechanics and Control of the Cytoskeleton in Amoeba Proteus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Viable Therapy Against Metastatic Breast Cancer
Graduate Theses, Dissertations, and Problem Reports 2017 Dual Targeting of the Mesenchymal and Amoeboid Pathways: A Viable Therapy against Metastatic Breast Cancer Brandon C. Jones Follow this and additional works at: https://researchrepository.wvu.edu/etd Recommended Citation Jones, Brandon C., "Dual Targeting of the Mesenchymal and Amoeboid Pathways: A Viable Therapy against Metastatic Breast Cancer" (2017). Graduate Theses, Dissertations, and Problem Reports. 5905. https://researchrepository.wvu.edu/etd/5905 This Dissertation is protected by copyright and/or related rights. It has been brought to you by the The Research Repository @ WVU with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you must obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Dissertation has been accepted for inclusion in WVU Graduate Theses, Dissertations, and Problem Reports collection by an authorized administrator of The Research Repository @ WVU. For more information, please contact [email protected]. Dual Targeting of the Mesenchymal and Amoeboid Pathways: A Viable Therapy against Metastatic Breast Cancer Brandon C. Jones Dissertation submitted to the School of Medicine at West Virginia University in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biochemistry & Molecular Biology Elena Pugacheva, Ph.D., Chair J. Michael Ruppert, M.D., Ph.D. Maxim Sokolov, Ph.D. Michael Schaller, Ph.D. Scott Weed, Ph.D. -

Emergence of Self-Organized Amoeboid Movement in a Multi-Agent Approximation of Physarum Polycephalum
Emergence of Self-Organized Amoeboid Movement in a Multi-Agent Approximation of Physarum polycephalum Jeff Jones and Andrew Adamatzky Centre for Unconventional Computing, University of the West of England, Coldharbour Lane, Bristol BS16 1QY, UK. [email protected] , [email protected] Abstract: The giant single-celled slime mould Physarum polycephalum exhibits complex morphological adaptation and amoeboid movement as it forages for food and may be seen as a minimal example of complex robotic behaviour. Swarm computation has previously been used to explore how spatio- temporal complexity can emerge from, and be distributed within, simple component parts and their interactions. Using a particle based swarm approach we explore the question of how to generate collective amoeboid movement from simple non-oscillatory component parts in a model of P. polycephalum . The model collective behaves as a cohesive and deformable virtual material, approximating the local coupling within the plasmodium matrix. The collective generates de-novo and complex oscillatory patterns from simple local interactions. The origin of this motor behaviour is distributed within the collective rendering is morphologically adaptive, amenable to external influence, and robust to simulated environmental insult. We show how to gain external influence over the collective movement by simulated chemo-attraction (pulling towards nutrient stimuli) and simulated light irradiation hazards ( pushing from stimuli). The amorphous and distributed properties of the collective are demonstrated by cleaving it into two independent entities and fusing two separate entities to form a single device, thus enabling it to traverse narrow, separate or tortuous paths. We conclude by summarising the contribution of the model to swarm based robotics and soft-bodied modular robotics and discuss the future potential of such material approaches to the field. -

Protozoa : Locomotion and Nutrition
Protozoa : locomotion and Nutrition Lecturer : P. V. Deokar Department of Zoology R.K.M.M. Ahmednagar Locomotion in Protozoa • The following points highlight the three main types of locomotion exhibited by protozoans. The types of locomotion are: • 1. Amoeboid Movement • 2. Flagellar Movement • 3. Ciliary Movement. Protozoans: Type of Locomotion # 1. Amoeboid Movement: • movement of the animal is made by the throwing of pseudopodium, called amoeboid movement • In the direction of movement of Amoeba a new pseudopodium is formed and the pseudopodium at the opposite side gradually disappears. • Types of pseudopodia: • According to form, structure and activity four different kinds of pseudopodia are recognised • These are: • (a) Lobopodium • (b) Filopodium • (c) Reticulopodium or Rhizopodium • (d) Axopodium or Actinopodium (a) Lobopodium [Gk. lobes = lobe; podium = foot]: • It is a short, finger or tongue-like projection which is accompanied by a flow of endoplasm and ectoplasm. • The pseudopodium is broad with rounded or blunt tips. • The ectoplasmmic area is distinctly clear, called the hyaline cap. • It is the characteristic of many amoebas such as Amoeba. (b) Filopodium [L.filo = a thread; podium = foot]: • The filopodium is a slender, thread-like or filamentous projection. • It is formed by the ectoplasm alone and without a hyaline cap. • The filaments are narrow and may be branched but do not anastomose, Filopodium is the characteristic in Filosea (e.g., Euglypha ). (c) Reticulopodium or Rhizopodium [L. reticulos = a net, podium = foot]: • Similar in structure to that of filopodium but the branches anastomose. • The numerous branched and anastomosed pseudopodia form a dense network, help primarily in capturing the prey and the secondary function is locomotion. -

Acanthamoeba Is an Evolutionary Ancestor of Macrophages: a Myth Or Reality? Ruqaiyyah Siddiqui Aga Khan University
eCommons@AKU Department of Biological & Biomedical Sciences Medical College, Pakistan February 2012 Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality? Ruqaiyyah Siddiqui Aga Khan University Naveed Ahmed Khan Aga Khan University Follow this and additional works at: http://ecommons.aku.edu/pakistan_fhs_mc_bbs Part of the Biochemistry Commons Recommended Citation Siddiqui, R., Khan, N. (2012). Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality?. Experimental Parasitology, 130(2), 95-97. Available at: http://ecommons.aku.edu/pakistan_fhs_mc_bbs/16 Experimental Parasitology 130 (2012) 95–97 Contents lists available at SciVerse ScienceDirect Experimental Parasitology journal homepage: www.elsevier.com/locate/yexpr Minireview Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality? ⇑ Ruqaiyyah Siddiqui a, Naveed Ahmed Khan a,b, a Aga Khan University, Stadium Road, Karachi, Pakistan b School of Veterinary Medicine and Science, University of Nottingham, Sutton Bonington, England, UK article info abstract Article history: Given the remarkable similarities in cellular structure (morphological and ultra-structural features), Received 26 October 2011 molecular motility, biochemical physiology, ability to capture prey by phagocytosis and interactions with Received in revised form 17 November 2011 microbial pathogens, here we pose the question whether Acanthamoeba and macrophages are evolution- Accepted 19 November 2011 ary related. This is discussed in the light of evolution and functional aspects such as the astonishing Available online 28 November 2011 resemblance of many bacteria to infect and multiply inside human macrophages and amoebae in analo- gous ways. Further debate and studies will determine if Acanthamoeba is an evolutionary ancestor of Keywords: macrophages. Is this a myth or reality? Acanthamoeba Ó 2011 Elsevier Inc. -
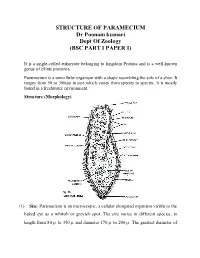
STRUCTURE of PARAMECIUM Dr Poonam Kumari Dept of Zoology (BSC PART I PAPER I)
STRUCTURE OF PARAMECIUM Dr Poonam kumari Dept Of Zoology (BSC PART I PAPER I) It is a single-celled eukaryote belonging to kingdom Protista and is a well-known genus of ciliate protozoa. Paramecium is a unicellular organism with a shape resembling the sole of a shoe. It ranges from 50 to 300um in size which varies from species to species. It is mostly found in a freshwater environment. Structure (Morphology) (1) Size: Paramecium is an microscopic, a cellular elongated organism visible to the baked eye as a whitish or greyish spot. The size varies in different species, in length from 80 to 350 and diameter 170 to 290 . The greatest diameter of the cylindrical body is about two third of its entire length. Usually the individuals of the same species may show minor morphological and physiological differences. (2) Shape: Paramecium is a slipper shaped, cigar shaped, or spindle shaped animalcule. Its shape is usually constant and a symmetrical, because slipper like shape. The body is elongated, blunt and rounded at the anterior end and somewhat pointed of the posterior end. In cross section it is circular with greatest diameter behind the centre of body. The anterior half of the body is slightly twisted. The body is distinguished into an oral or ventral surface and an aboral or dorsal surface. The structure is more complicated due to the development of certain organelles in the acellular body. (3) Oral groove: The ventral surface of body bears a prominent, oblique and shallow depression is called oral groove, it arise from the middle of body and extends to the left side of anterior end. -

On the Physiology of Amoeboid Movement
ON THE PHYSIOLOGY OF AMOEBOID MOVEMENT. IV.—THE ACTION OF MAGNESIUM. BY C. F. A. PANTIN. {The Marine Biological Laboratory, Plymouth?) (Received January 6th, 1926.) CONTENTS 1. Maintenance of the cell-surface . 297 6. The action of Ce'" in the pres- 2. The action of barium . 300 ence of Ca" .... 306 3. The action of cerium . 301 4. Action of Mg" in the presence 7. Discussion 306 of Ca" 302 8. Summary 310 5. Interaction of the ions of sea- 9. References 311 water 303 THE previous paper of this series (Pantin, 1926) described the action of certain ions upon a species of marine amoeba, and especially the relation of calcium to amoeboid movement. The same paper detailed the method of preparing isotonic salt solutions and of determining their effect on amoeboid movement. The essential feature is that the average velocity is taken as a measure of the effect of a solution upon the power of movement, apart from other effects produced upon the cell. Unless otherwise stated, the CH of the solutions was maintained at /H 7.0 to 7.2. 1. Maintenance of the cell-surface. It was shown previously that whereas cytolysis occurred rapidly in pure isotonic NaCl or KCl (at about />H 7), amoebae remained alive for a longer time in isotonic CaCl= and MgClj. Movement was inhibited in these solutions, but reversibly, for, provided immersion had been brief, recovery occurred on return to natural sea-water. In mixtures of two salts, it was found that movement only occurred if Ca were 297 C. F. A. -

EE Just's "Independent Irritability"
ESSAY Molecular Reproduction & Development 76:966–974 (2009) E.E. Just’s ‘‘Independent Irritability’’ Revisited: The Activated Egg as Excitable Soft Matter STUART A. NEWMAN* Department of Cell Biology and Anatomy, New York Medical College, Valhalla, New York SUMMARY Ernest Everett Just’s experimental work on post-fertilization events in invertebrate eggs led him to posit a dynamic and directive role for the zygotic ‘‘ectoplasm’’ (cortical Just was correct in his estimation cytoplasm), in subsequent development. His perspective was neglected during the of the ‘‘informational’’ role of the years that followed his early death not only because of his well-documented margina- ectoplasm’s dynamics. lization as an African-American in U.S. science, but because his ideas were at odds with the growing gene-centrism of developmental biology in the latter half of the 20th century. This essay reviews experimental work that shows that the egg cortex in many animal groups is a chemically and mechanically active medium that sustains both spatiotemporal calcium ion transients and periodic deformations in the time leading up * Corresponding author: to cleavage. These wave phenomena are seen to play regulatory roles in germ plasm Department of Cell Biology and localization and gene expression, and influence the reliability and success of devel- Anatomy opmental outcomes. Just resisted vitalistic explanations for the active processes he New York Medical College Basic Science Building observed and inferred regarding the egg cortical cytoplasm, but recognized that the Valhalla, NY 10595. physics and chemistry of his time were inadequate to account for these phenomena E-mail: [email protected] and anticipated that expansions of these fields would be necessary to explain them. -

Cytoskeleton and Cell Motility
Cytoskeleton and Cell Motility 1. (28 pts) Amoeba proteus, a single-celled eukaryote, moves by means of psudopods attaching to and detaching from the substratum. Locomotion seems to be correlated with the forward flow of fluid cytoplasm (endoplasm) into an advancing pseudopod through a surrounding, gel-like ectoplasmic tube. The ectoplasm forms at the pseudopodial tip in a region called the Fountain Zone. As the amoeba advances the ectoplasmic tube “liquifies” at the posterior end to form endoplasm. These features are illustrated in the figure below. Both locomotion and cytoplasmic streaming are inhibited by cytochalasin B. Consider everything you’ve learned so far and answer all the following questions. A. (4 pts) When amoeba undergoes cell division, it stops streaming and rounds up into a spherical cell. Describe how this change in shape and behavior comes about and why it might be a necessary precondition for division. B. (6 pts) Briefly describe how cytoplasmic streaming is most likely organized and generated at the cellular and molecular levels. C. (5 pts) Briefly describe an additional experiment or observation that would test your hypothesis and indicate clearly what the results would show. CCM - 1 Cytoskeleton and Cell Motility D. (8 pts) Describe clearly, with the aid of a well-labeled diagram, how streaming within a pseudopod could result in movement of the amoeba across the substratum. E. (5 pts) Describe how your streaming mechanism might be regulated such that the amoeba might change its streaming pattern to form phagocytic pseudopods around a ciliate it had touched. Now evaluate some past answers to these questions, in light of your own essays. -

Structure and Development of the Egg of the Glossiphoniid Leech Theromyzon Rude: Characterization of Developmental Stages and Structure of the Early Uncleaved Egg
Development 100, 211-225 (1987) 211 Printed in Great Britain © The Company of Biologists Limited 1987 Structure and development of the egg of the glossiphoniid leech Theromyzon rude: characterization of developmental stages and structure of the early uncleaved egg JUAN FERNANDEZ, NANCY OLEA and CECILIA MATTE Departamento de Biologia, Facultad de Ciendas, Untversidad de Chile, Casilla 653, Santiago, Chile Summary Some aspects of the reproductive biology of the meridional bands, during stage le, lead to accumu- glossiphoniid leech, Theromyzon rude, under labora- lation of ooplasm at both egg poles. In this manner, tory conditions, and the staging and structure of its the teloplasm or pole plasm forms. Completion of the uncleaved egg were studied. Sexually mature animals first cleavage furrow, by the end of stage If, is form breeding communities and fertilization occurs in preceded by dorsoventral flattening of the egg and the ovLsacs, presumably around the time of egg rearrangement of its teloplasm and perinuclear laying. Opposition may be postponed for hours or plasm. Structure of the early uncleaved egg has been days, but the eggs in the ovisacs remain blocked at studied with transmission and scanning electron mi- first meiotic metaphase. Development of the croscopy of intact or permeabilized preparations. The uncleaved egg, from the time of oviposit ion to com- plasmalemma forms numerous long and some short pletion of the first cleavage division, has been sub- microvilli evenly distributed across the egg surface. divided into six stages. At 20 °C, the six developmental The ectoplasm includes many vesicles, mitochondria, stages take 5-6 h. Characterization of' the stages is granules and an elaborate network of filament based on observations of both live and fixed/cleared bundles. -

Protists: Amoeba
Microorganisms – Protists: Amoeba The amoeba is a protozoan that belongs to the Kingdom Protista. The name amoeba comes from the Greek word amoibe, which means change. (Amoeba is also spelled ameba.) Protists are microscopic unicellular organisms that don't fit into the other kingdoms. Some protozoans are considered plant- like while others are considered animal-like. The amoeba is considered an animal-like protist because it moves and consumes its food. Protists are classified by how they move, some have cilia or flagella, but the ameba has an unusual way of creeping along by stretching its cytoplasm into fingerlike extensions called pseudopodia. The word "pseudopodia" means "false foot". Label the pseudopodia. When looking at ameba under a microscope, an observer will note that no amoeba looks the same as any other. The cell membrane is very flexible and allows for the amoeba to change shape. Amoebas live in ponds or puddles, and some can even live inside people. Color and label the cell membrane red. There are two types of cytoplasm in the amoeba. The darker cytoplasm toward the interior of the protozoan is called endoplasm. The clearer cytoplasm that is found near the cell membrane is called ectoplasm. On the diagram, the endoplasm is indicated by the dotted area, and the ectoplasm by the white area. Color and label the endoplasm pink. Color and label the ectoplasm light blue. By pushing the endoplasm toward the cell membrane, the amoeba causes its body to extend and creep along. It is also by this method that the amoeba consumes its food. -

Difference Between Ectoplasm and Endoplasm Key Difference – Ectoplasm Vs Endoplasm
Difference Between Ectoplasm and Endoplasm www.differencebetween.com Key Difference – Ectoplasm vs Endoplasm Protozoa are single-cell eukaryotic organisms. They resemble animal cells and contain major organelles and the cell nucleus. Protozoan’s cytoplasm has two distinct areas called ectoplasm and endoplasm. The outer layer of the cytoplasm is known as the ectoplasm. The inner layer is known as the endoplasm. The terms endoplasm and ectoplasm are used mainly to describe amoeba cytoplasm and how it helps feeding and locomotion. Amoeba is a single cell eukaryotic organism which is made up of a nucleus and cytoplasm. The cytoplasm of amoeba can be divided into the two layers: endoplasm and ectoplasm. Ectoplasm is the clear outer cytoplasmic layer of amoeba while endoplasm is the inner granule-rich cytoplasmic layer of amoeba. This is the key difference between ectoplasm and endoplasm. What is Ectoplasm? Ectoplasm refers to the outer layer of the cytoplasm of a cell. It is not a granulated area. This part of the cytoplasm is watery and clear. Ectoplasm is located immediately adjacent to the plasma membrane. It is clearly visible in the amoeba cell. Figure 01: Cell Structure Amoeba Amoeba cells locomote by pseudopodia formation. The ectoplasm of the amoeba cell is responsible for changing the direction of the pseudopodium. Location of the pseudopodium changes when the alkalinity and acidity of the water in ectoplasm are changed. A slight change in the acidity or alkalinity is enough for the flowing of cytoplasm which helps in locomotion. Water concentration of the amoeba cell is regulated by the endoplasm. Endoplasm easily absorbs or releases water through the partially permeable membrane. -

Studies on the Organization and Localization of Actin and Myosin in Neurons
STUDIES ON THE ORGANIZATION AND LOCALIZATION OF ACTIN AND MYOSIN IN NEURONS EDWARD R. KUCZMARSKI and JOEL L. ROSENBAUM From the Department of Biology, Yale University, New Haven, Connecticut 06520 ABSTRACT The organization of actin in mouse neuroblastoma and chicken dorsal root ganglion (DRG) nerve cells was investigated by means of a variety of electron microscope techniques. Microspikes of neuroblastoma cells contained bundles of 7- to 8-nm actin filaments which originated in the interior of the neurite. In the presence of high concentrations of Mg ++ ion, filaments in these bundles became highly ordered to form paracrystals. Actin filaments, but not bundles, were observed in growth cones of DRG cells. Actin was localized in the cell body, neurites, and microspikes ot~ both DRG and neuroblastoma nerve cells by fluorescein-labeled S1. Myosin was localized primarily in the neurites of chick DRG nerve cells with fluorescein-labeled anti-brain myosin antibody. This antibody also stained stress fibers in fibroblasts and myoblasts but did not stain muscle myofibrils. KEY WORDS neurons microspikes poisons stops the transport and causes a piling up microfilaments myosin localization of material proximal to the block (44). A retro- grade transport has also been described in which Neurons exhibit a variety of motile phenomena, material moves from the axon tip toward the cell one of the most dramatic examples being nerve body (35). Some form of motility may also be outgrowth. In experiments which proved the neu- required for transmitter release at the synapse (4). ron theory, Harrison (28) first described the rapid These examples demonstrate that several nerve movement of nerve cells in tissue culture where functions involve motility.