Actomyosin Content of Physarum Plasmodia and Detection of Immunological Cross-Reactions with Myosins from Related Species
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Public Description of Physarum Polycephalum Schwein. Public Description of Physarum Polycephalum Schwein
4/27/2017 Mushroom Observer: Public Description of Physarum polycephalum Schwein. Public Description of Physarum polycephalum Schwein. Title: Public Description (Default) Descriptions: Create Name: Physarum polycephalum Schwein. (/name/create_name_description/30400) View: public Public Description (Default) Edit: public (/name/show_name_description/1634) [Edit Version: 2 (/name/edit_name_description/1634)] Previous Version (/name/show_past_name_description/1634? version=1) Description status: Unreviewed Taxonomic Classification: Kingdom: Amoebozoa (http://mushroomobserver.org/observer/lookup_name/Amoebozoa) Phylum: Mycetozoa (http://mushroomobserver.org/observer/lookup_name/Mycetozoa) Class: Myxogastria (http://mushroomobserver.org/observer/lookup_name/Myxogastria) Order: Physarida (http://mushroomobserver.org/observer/lookup_name/Physarida) Family: Physaridae (http://mushroomobserver.org/observer/lookup_name/Physaridae) Genus: Physarum (http://mushroomobserver.org/observer/lookup_name/Physarum) General Description: Physarium polycephalum is one of the most widely recognized and cultivated plasmodia. P. polycephalum undergoes distinctive transformations from spore, to amoeboid, to plasmoid and sporangium. To the naked eye, the spores appear as a reddish or purplish brown mass; amoebas and myxoflagellates can only be seen under microscope; the plasmodium will be a bright yellow, highly reticulate network and have a much finer, denser, distinctive “fan” shape in the direction of movement. Sporangia are dark orange to brown and consist of gregarious lobes connected to a shriveled, thin stipe. Spores are uninucleate, globose, minutely spinose, 8 to 11 microns in diameter, brownish yellow individually, appear reddish to purplish brown in mass, and require 15 to 48 hours for incubation, defined as the time when seeds are sowed to the splitting of the spore walls. Incubation duration is constant whether a spore is fresh or 18 months old. Cultures are readily started on water drops on the surface of slides. -

Old Woman Creek National Estuarine Research Reserve Management Plan 2011-2016
Old Woman Creek National Estuarine Research Reserve Management Plan 2011-2016 April 1981 Revised, May 1982 2nd revision, April 1983 3rd revision, December 1999 4th revision, May 2011 Prepared for U.S. Department of Commerce Ohio Department of Natural Resources National Oceanic and Atmospheric Administration Division of Wildlife Office of Ocean and Coastal Resource Management 2045 Morse Road, Bldg. G Estuarine Reserves Division Columbus, Ohio 1305 East West Highway 43229-6693 Silver Spring, MD 20910 This management plan has been developed in accordance with NOAA regulations, including all provisions for public involvement. It is consistent with the congressional intent of Section 315 of the Coastal Zone Management Act of 1972, as amended, and the provisions of the Ohio Coastal Management Program. OWC NERR Management Plan, 2011 - 2016 Acknowledgements This management plan was prepared by the staff and Advisory Council of the Old Woman Creek National Estuarine Research Reserve (OWC NERR), in collaboration with the Ohio Department of Natural Resources-Division of Wildlife. Participants in the planning process included: Manager, Frank Lopez; Research Coordinator, Dr. David Klarer; Coastal Training Program Coordinator, Heather Elmer; Education Coordinator, Ann Keefe; Education Specialist Phoebe Van Zoest; and Office Assistant, Gloria Pasterak. Other Reserve staff including Dick Boyer and Marje Bernhardt contributed their expertise to numerous planning meetings. The Reserve is grateful for the input and recommendations provided by members of the Old Woman Creek NERR Advisory Council. The Reserve is appreciative of the review, guidance, and council of Division of Wildlife Executive Administrator Dave Scott and the mapping expertise of Keith Lott and the late Steve Barry. -

Slime Moulds
Queen’s University Biological Station Species List: Slime Molds The current list has been compiled by Richard Aaron, a naturalist and educator from Toronto, who has been running the Fabulous Fall Fungi workshop at QUBS between 2009 and 2019. Dr. Ivy Schoepf, QUBS Research Coordinator, edited the list in 2020 to include full taxonomy and information regarding species’ status using resources from The Natural Heritage Information Centre (April 2018) and The IUCN Red List of Threatened Species (February 2018); iNaturalist and GBIF. Contact Ivy to report any errors, omissions and/or new sightings. Based on the aforementioned criteria we can expect to find a total of 33 species of slime molds (kingdom: Protozoa, phylum: Mycetozoa) present at QUBS. Species are Figure 1. One of the most commonly encountered reported using their full taxonomy; common slime mold at QUBS is the Dog Vomit Slime Mold (Fuligo septica). Slime molds are unique in the way name and status, based on whether the species is that they do not have cell walls. Unlike fungi, they of global or provincial concern (see Table 1 for also phagocytose their food before they digest it. details). All species are considered QUBS Photo courtesy of Mark Conboy. residents unless otherwise stated. Table 1. Status classification reported for the amphibians of QUBS. Global status based on IUCN Red List of Threatened Species rankings. Provincial status based on Ontario Natural Heritage Information Centre SRank. Global Status Provincial Status Extinct (EX) Presumed Extirpated (SX) Extinct in the -

Biodiversity of Plasmodial Slime Moulds (Myxogastria): Measurement and Interpretation
Protistology 1 (4), 161–178 (2000) Protistology August, 2000 Biodiversity of plasmodial slime moulds (Myxogastria): measurement and interpretation Yuri K. Novozhilova, Martin Schnittlerb, InnaV. Zemlianskaiac and Konstantin A. Fefelovd a V.L.Komarov Botanical Institute of the Russian Academy of Sciences, St. Petersburg, Russia, b Fairmont State College, Fairmont, West Virginia, U.S.A., c Volgograd Medical Academy, Department of Pharmacology and Botany, Volgograd, Russia, d Ural State University, Department of Botany, Yekaterinburg, Russia Summary For myxomycetes the understanding of their diversity and of their ecological function remains underdeveloped. Various problems in recording myxomycetes and analysis of their diversity are discussed by the examples taken from tundra, boreal, and arid areas of Russia and Kazakhstan. Recent advances in inventory of some regions of these areas are summarised. A rapid technique of moist chamber cultures can be used to obtain quantitative estimates of myxomycete species diversity and species abundance. Substrate sampling and species isolation by the moist chamber technique are indispensable for myxomycete inventory, measurement of species richness, and species abundance. General principles for the analysis of myxomycete diversity are discussed. Key words: slime moulds, Mycetozoa, Myxomycetes, biodiversity, ecology, distribu- tion, habitats Introduction decay (Madelin, 1984). The life cycle of myxomycetes includes two trophic stages: uninucleate myxoflagellates General patterns of community structure of terrestrial or amoebae, and a multi-nucleate plasmodium (Fig. 1). macro-organisms (plants, animals, and macrofungi) are The entire plasmodium turns almost all into fruit bodies, well known. Some mathematics methods are used for their called sporocarps (sporangia, aethalia, pseudoaethalia, or studying, from which the most popular are the quantita- plasmodiocarps). -

9B Taxonomy to Genus
Fungus and Lichen Genera in the NEMF Database Taxonomic hierarchy: phyllum > class (-etes) > order (-ales) > family (-ceae) > genus. Total number of genera in the database: 526 Anamorphic fungi (see p. 4), which are disseminated by propagules not formed from cells where meiosis has occurred, are presently not grouped by class, order, etc. Most propagules can be referred to as "conidia," but some are derived from unspecialized vegetative mycelium. A significant number are correlated with fungal states that produce spores derived from cells where meiosis has, or is assumed to have, occurred. These are, where known, members of the ascomycetes or basidiomycetes. However, in many cases, they are still undescribed, unrecognized or poorly known. (Explanation paraphrased from "Dictionary of the Fungi, 9th Edition.") Principal authority for this taxonomy is the Dictionary of the Fungi and its online database, www.indexfungorum.org. For lichens, see Lecanoromycetes on p. 3. Basidiomycota Aegerita Poria Macrolepiota Grandinia Poronidulus Melanophyllum Agaricomycetes Hyphoderma Postia Amanitaceae Cantharellales Meripilaceae Pycnoporellus Amanita Cantharellaceae Abortiporus Skeletocutis Bolbitiaceae Cantharellus Antrodia Trichaptum Agrocybe Craterellus Grifola Tyromyces Bolbitius Clavulinaceae Meripilus Sistotremataceae Conocybe Clavulina Physisporinus Trechispora Hebeloma Hydnaceae Meruliaceae Sparassidaceae Panaeolina Hydnum Climacodon Sparassis Clavariaceae Polyporales Gloeoporus Steccherinaceae Clavaria Albatrellaceae Hyphodermopsis Antrodiella -

Slime Molds: Biology and Diversity
Glime, J. M. 2019. Slime Molds: Biology and Diversity. Chapt. 3-1. In: Glime, J. M. Bryophyte Ecology. Volume 2. Bryological 3-1-1 Interaction. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 18 July 2020 and available at <https://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 3-1 SLIME MOLDS: BIOLOGY AND DIVERSITY TABLE OF CONTENTS What are Slime Molds? ....................................................................................................................................... 3-1-2 Identification Difficulties ...................................................................................................................................... 3-1- Reproduction and Colonization ........................................................................................................................... 3-1-5 General Life Cycle ....................................................................................................................................... 3-1-6 Seasonal Changes ......................................................................................................................................... 3-1-7 Environmental Stimuli ............................................................................................................................... 3-1-13 Light .................................................................................................................................................... 3-1-13 pH and Volatile Substances -

Acanthamoeba Is an Evolutionary Ancestor of Macrophages: a Myth Or Reality? Ruqaiyyah Siddiqui Aga Khan University
eCommons@AKU Department of Biological & Biomedical Sciences Medical College, Pakistan February 2012 Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality? Ruqaiyyah Siddiqui Aga Khan University Naveed Ahmed Khan Aga Khan University Follow this and additional works at: http://ecommons.aku.edu/pakistan_fhs_mc_bbs Part of the Biochemistry Commons Recommended Citation Siddiqui, R., Khan, N. (2012). Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality?. Experimental Parasitology, 130(2), 95-97. Available at: http://ecommons.aku.edu/pakistan_fhs_mc_bbs/16 Experimental Parasitology 130 (2012) 95–97 Contents lists available at SciVerse ScienceDirect Experimental Parasitology journal homepage: www.elsevier.com/locate/yexpr Minireview Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality? ⇑ Ruqaiyyah Siddiqui a, Naveed Ahmed Khan a,b, a Aga Khan University, Stadium Road, Karachi, Pakistan b School of Veterinary Medicine and Science, University of Nottingham, Sutton Bonington, England, UK article info abstract Article history: Given the remarkable similarities in cellular structure (morphological and ultra-structural features), Received 26 October 2011 molecular motility, biochemical physiology, ability to capture prey by phagocytosis and interactions with Received in revised form 17 November 2011 microbial pathogens, here we pose the question whether Acanthamoeba and macrophages are evolution- Accepted 19 November 2011 ary related. This is discussed in the light of evolution and functional aspects such as the astonishing Available online 28 November 2011 resemblance of many bacteria to infect and multiply inside human macrophages and amoebae in analo- gous ways. Further debate and studies will determine if Acanthamoeba is an evolutionary ancestor of Keywords: macrophages. Is this a myth or reality? Acanthamoeba Ó 2011 Elsevier Inc. -

The Mycetozoa of North America, Based Upon the Specimens in The
THE MYCETOZOA OF NORTH AMERICA HAGELSTEIN, MYCETOZOA PLATE 1 WOODLAND SCENES IZ THE MYCETOZOA OF NORTH AMERICA BASED UPON THE SPECIMENS IN THE HERBARIUM OF THE NEW YORK BOTANICAL GARDEN BY ROBERT HAGELSTEIN HONORARY CURATOR OF MYXOMYCETES ILLUSTRATED MINEOLA, NEW YORK PUBLISHED BY THE AUTHOR 1944 COPYRIGHT, 1944, BY ROBERT HAGELSTEIN LANCASTER PRESS, INC., LANCASTER, PA. PRINTED IN U. S. A. To (^My CJriend JOSEPH HENRI RISPAUD CONTENTS PAGES Preface 1-2 The Mycetozoa (introduction to life history) .... 3-6 Glossary 7-8 Classification with families and genera 9-12 Descriptions of genera and species 13-271 Conclusion 273-274 Literature cited or consulted 275-289 Index to genera and species 291-299 Explanation of plates 301-306 PLATES Plate 1 (frontispiece) facing title page 2 (colored) facing page 62 3 (colored) facing page 160 4 (colored) facing page 172 5 (colored) facing page 218 Plates 6-16 (half-tone) at end ^^^56^^^ f^^ PREFACE In the Herbarium of the New York Botanical Garden are the large private collections of Mycetozoa made by the late J. B. Ellis, and the late Dr. W. C. Sturgis. These include many speci- mens collected by the earlier American students, Bilgram, Farlow, Fullmer, Harkness, Harvey, Langlois, Macbride, Morgan, Peck, Ravenel, Rex, Thaxter, Wingate, and others. There is much type and authentic material. There are also several thousand specimens received from later collectors, and found in many parts of the world. During the past twenty years my associates and I have collected and studied in the field more than ten thousand developments in eastern North America. -

Physarum Polycephalum) by SPME
Analysis of the volatiles in the headspace above the plasmodium and sporangia of the slime mould (Physarum polycephalum) by SPME- GCMS Huda al Kateb1 and Ben de Lacy Costello1 1Institute for biosensing technology, University of the West of England, Bristol, BS161QY, UK E-mail: [email protected] Abstract Solid phase micro-extraction (SPME) coupled with Gas Chromatography Mass Spectrometry (GC-MS) was used to extract and analyse the volatiles in the headspace above the plasmodial and sporulating stages of the slime mould Physarum Polycephalum. In total 115 compounds were identified from across a broad range of chemical classes. Although more (87) volatile organic compounds (VOCs) were identified when using a higher incubation temperature of 75oC, a large number of compounds (79) were still identified at the lower extraction temperature of 30oC and where the plasmodial stage was living. Far fewer compounds were extracted after sporulation at the two extraction temperatures. There were some marked differences between the VOCs identified in the plasmodial stage and after sporulation. In particular the nitrogen containing compounds acetonitrile, pyrrole, 2, 5-dimethyl-pyrazine and trimethyl pyrazine seemed to be associated with the sporulating stage. There were many compounds associated predominantly with the plasmodial stage including a number of furans and alkanes. Interestingly, a number of known fungal metabolites were identified including 1-octen-3- ol, 3-octanone, 1-octen-3-one, 3-octanol. In addition known metabolites of cyanobacteria and actinobacteria in particular geosmin was identified in the headspace. Volatile metabolites that had previously been identified as having a positive chemotactic response to the plasmodial stage of P. -
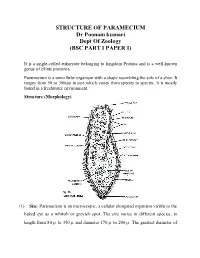
STRUCTURE of PARAMECIUM Dr Poonam Kumari Dept of Zoology (BSC PART I PAPER I)
STRUCTURE OF PARAMECIUM Dr Poonam kumari Dept Of Zoology (BSC PART I PAPER I) It is a single-celled eukaryote belonging to kingdom Protista and is a well-known genus of ciliate protozoa. Paramecium is a unicellular organism with a shape resembling the sole of a shoe. It ranges from 50 to 300um in size which varies from species to species. It is mostly found in a freshwater environment. Structure (Morphology) (1) Size: Paramecium is an microscopic, a cellular elongated organism visible to the baked eye as a whitish or greyish spot. The size varies in different species, in length from 80 to 350 and diameter 170 to 290 . The greatest diameter of the cylindrical body is about two third of its entire length. Usually the individuals of the same species may show minor morphological and physiological differences. (2) Shape: Paramecium is a slipper shaped, cigar shaped, or spindle shaped animalcule. Its shape is usually constant and a symmetrical, because slipper like shape. The body is elongated, blunt and rounded at the anterior end and somewhat pointed of the posterior end. In cross section it is circular with greatest diameter behind the centre of body. The anterior half of the body is slightly twisted. The body is distinguished into an oral or ventral surface and an aboral or dorsal surface. The structure is more complicated due to the development of certain organelles in the acellular body. (3) Oral groove: The ventral surface of body bears a prominent, oblique and shallow depression is called oral groove, it arise from the middle of body and extends to the left side of anterior end. -

Checklists of the Myxomycetes, Larger Ascomycetes, and Larger
Posted online: january 2009 “This internet site was updated on January 2011” Summary published in MYCOTAXON 106: 65-68. Sesli, E. and Denchev, CM. (2008). Checklists of the myxomycetes, larger ascomycetes, and larger basidiomycetes in Turkey. Mycotaxon 106: 65–67 + online version [2011]: 1-136 (http://www.mycotaxon.com/resources/checklists/sesli-v106-checklist.pdf) Checklists of the myxomycetes, larger ascomycetes, and larger basidiomycetes in Turkey 1 2 Ertuğrul Sesli & Cvetomir M. Denchev 1 Department of Biology Education, Karadeniz Technical University, Trabzon, Turkey (e-mail: [email protected]) 2 Institute of Botany, Bulgarian Academy of Sciences, 23 Acad. G. Bonchev St., 1113 Sofia, Bulgaria (e-mail: [email protected]) Corresponding author: [email protected] Sesli & Denchev – Checklists of the myxomycetes, larger ascomycetes, and larger basidiomycetes in Turkey 2 Abstract This paper attempts to compile available data on myxomycetes, larger ascomycetes, and larger basidiomycetes reported from or known to occur in Turkey, obtained from 428 publications issued between 1915 and January, 2011. Three main lists of correct names of myxomycetes, larger ascomycetes, and larger basidiomycetes, recognized as occurring in Turkey, are given, in which the taxa are alphabetically arranged. The total number of correct names of species, recognized as occurring in Turkey and presented in the checklists, is 2196, including 222 myxomycetes, 152 ascomycetes, and 1822 basidiomycetes. For each taxon, references are cited. An index of synonyms based on literature records from Turkey is appended. The index includes 890 species and infraspecific taxa. A list of excluded records of 80 species, providing reasons for their exclusion, is also given. -

The Myxomycetes of Athens Conty, Ohio
The Myxomycetes of Athens County, Ohio1 DAKKIN L. RUIJINO2 AND JAMKS C. CAVI;NI)KR, Department of Environmental and Plant Biology, Ohio University, Athens, OH 45701 ABSTRACT. The goal of this study was to document all reported collections of myxomycetes (slime molds) from Athens County, OH (USA). The compilation of several published and unpublished studies of myxomycete records from Athens County resulted in a total of 52 species. The species were distributed among 6 orders, 9 families, and 25 genera and represent 24% of the myxomycetes known from Ohio and approximately 15% of those recorded for North America. No new collections for the state of Ohio were reported. OHIO J SCI 102 (2):27-29, 2002 INTRODUCTION both authors. Nomenclature follows that of Keller and Although widely distributed, myxomycetes (true slime Braun (1999) [which closely follows the treatment of molds, acellular slime molds, or plasmodial slime molds) Martin and others (1983) and the synonymy of Martin have not been fully studied throughout Ohio. Despite and Alexopoulos (1969)]- major taxonomic works by Fulmer (1921) and Keller Collections made by Udall (1951) were from a beech- and Braun (1999), the distribution and ecology of the maple forest in Lee Township, and collections made by myxomycetes in several Ohio counties are not well Jones (1943) were from Athens Township (mesic forests known. For example, of the 88 counties in the state, only and Ohio University Campus). Jones' and Udall's theses 64 have recorded myxomycete collections, and many of are available from the Department of Environmental these counties have fewer than five recorded species and Plant Biology, Ohio University, Athens, OH.