Química (2016) 27, 175---181
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
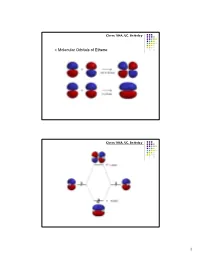
Π Molecular Orbitals of Ethene
Chem 104A, UC, Berkeley Molecular Orbitals of Ethene Chem 104A, UC, Berkeley 1 Chem 104A, UC, Berkeley Molecular Orbital Analysis of Ethene Dimerisation the reaction is said to be a "symmetry forbidden" – interestingly, this reaction is rare and very slow ! Chem 104A, UC, Berkeley Molecular Orbitals of 1,3-Butadiene 2 Chem 104A, UC, Berkeley Chem 104A, UC, Berkeley Molecular Orbital Analysis of Diels-Alder reaction 3 Chem 104A, UC, Berkeley Acid-Base Chemistry Reading : MT 6 Chem 104A, UC, Berkeley The Acid Base Theory of Brønsted and Lowry In 1923, within several months of each other, Johannes Nicolaus Brønsted (Denmark) and Thomas Martin Lowry (England) published essentially the same theory about how acids and bases behave. •An acid is a "proton donor." •A base is a "proton acceptor." 4 Chem 104A, UC, Berkeley + HCl + H2O <===> H3O + Cl¯ HCl - this is an acid, because it has a proton available to be transfered. H2O - this is a base, since it gets the proton that the acid lost. Now, here comes an interesting idea: + H3O - this is an acid, because it can give a proton. Cl¯ - this is a base, since it has the capacity to receive a proton. + Notice that each pair (HCl and Cl¯ as well as H2O and H3O differ by one proton (symbol = H+). These pairs are called conjugate pairs. Chem 104A, UC, Berkeley HCl + NaOH ---> H2O+ NaCl stronger stronger Weaker weaker conjugate conjugate acid base acid base + - NH4 +OH---> H2O+ NH3 stronger stronger weaker weaker conjugate conjugate acid base acid base 5 Chem 104A, UC, Berkeley H n A H 2O H n1 A H3O [H n1 A ][H3O ] pKa1 log [H n A] pKa 0 strong pKa 0 weak Chem 104A, UC, Berkeley Amphoteric Compound H n A H 2O H n1 A H3O NH 3 H 2O NH 4 OH 6 Chem 104A, UC, Berkeley Oxyacids AOp(OH)q A=Si, N, P, As, S, Se, Te, Cl, Br, I p=# of nonhydrogenated oxygen atom H + A ----O As atom A is rendered more positive, it becomes easier to break the O-H bond Because of enhanced bond polarization. -

Notes Acids and Bases
Notes Acids and Bases Arrhenius Acid Base Theory: In 1884 Svante Arrhenius proposed the first theoretical model for acids and bases. Prior to that time, these chemically opposite substances were described in properties such as their taste; their effects on metals, carbonates, and dyes (called indicators); their feel to the touch, and their ability to react with each other. According to the Arrhenius theory , pure water dissociates to some extent to produce hydrogen ions, H + and hydroxide ions, OH -. When this occurs, equal amounts of H + and OH - ions are produced: + - H2O(l) H (aq) + OH (aq) An acid, according to Arrhenius, is any substance that liberates H + ions when placed in water. When the H + concentration is elevated the OH - concentration decreases, this solution is said to be acidic. Similarly, a base is defined as any substance that liberates OH - ions when placed in water. The resulting solution has a higher concentration of OH - ions than H + ions and is said to be basic, or alkaline. + - ACID: HCl (aq) H (aq) + Cl (aq) + - BASE: NaOH (aq) Na (aq) + OH (aq) Brønsted - Lowry Acid Base Theory: A more general and realistic theory was proposed by two chemists working independent of the other, Johannes Nicolaus Brønsted of Denmark and Thomas Martin Lowry of England. Both chemists are given credit; the theory is named the Brønsted-Lowry theory . Acids are defined as substances that donate H + ions, protons and therefore are called proton donators, while bases are substances that accept protons and are defined as proton acceptors. On this basis, the autoionization of water is given as follows: + - H2O(l) + H 2O(l) H3O (aq) + OH (aq) In this reaction, one water molecule donates a proton, H +, and behaves as an acid, while the other water molecule accepts the proton and behaves as a base. -

History and the Teaching of Chemistry. a Tribute to Thomas Lowry's Textbook
Educación Química (2016) 27, 175---181 educación Química www.educacionquimica.info REFLECTION History and the teaching of chemistry. A tribute to Thomas Lowry’s textbook ‘‘Historical Introduction to Chemistry’’ William B. Jensen Department of Chemistry, University of Cincinnati, Cincinnati, OH 45221-0172, United States Received 9 March 2016; accepted 30 March 2016 Available online 11 June 2016 KEYWORDS Abstract Through Thomas Lowry’s book written in 1915, Historical Introduction to Chemistry, Words history this paper shows how chemistry can be taught from its own history. of chemistry; All Rights Reserved © 2016 Universidad Nacional Autónoma de México, Facultad de Química. Chemistry teaching; This is an open access item distributed under the Creative Commons CC License BY-NC-ND 4.0. Historical approach PALABRAS CLAVE Historia y Ensenanza˜ de la Química. Un tributo al libro de Thomas Lowry Historia de la ‘‘Introducción Histórica a la Química’’ química; Resumen A través del libro de Thomas Lowry escrito en 1915, Historical Introduction to Chem- Ensenanza˜ de la química; istry, este artículo muestra la manera de cómo se puede ensenar˜ química a partir de su propia Aproximación historia. histórica Derechos Reservados © 2016 Universidad Nacional Autónoma de México, Facultad de Química. Este es un artículo de acceso abierto distribuido bajo los términos de la Licencia Creative Commons CC BY-NC-ND 4.0. Though much has been written advocating the use of his- introductory textbooks which claim to include some his- tory of chemistry in the teaching of introductory chemistry tory of chemistry, usually in the form of portraits of select courses at both the high school and college levels, there chemists from the past with short biographical captions have been surprisingly few attempts to write a proper text- attached or, in a few cases, supplementary boxes briefly book based on this approach. -

J. R. Partington (1886-1965): Physical Chemistry in Deed and Word
Bull. Hist. Chem., VOLUME 34, Number 1 (2009) 11 J. R. PARTINGTON (1886-1965): PHYSICAL CHEMISTRY IN DEED AND WORD William H. Brock, University of Leicester, UK Introduction Scottish tailor. While he was still quite young his parents moved to the seaside town of Southport, to the north of Today James Riddick Partington (1886-1965) is remem- Liverpool, allowing Partington the benefit of education bered as an historian of chemistry rather than as the at the Victoria Science and Art School that had opened significant British research chemist and textbook writer in 1887 (1). Here his prowess as a mathematician and he was perceived to be in the 1920s and 1930s. Because practical chemist must have been forged. He left school his textbooks were specifically geared to the British in 1901 when he was 15 because his parents moved back secondary school and university systems, he is prob- to Bolton. There he began to assist the town’s Public ably not well known in the United States as a textbook Analyst, a post that must have involved the acquirement writer. Nor, in America or in Europe, is he remembered of the skills in volumetric and gravimetric analysis that as a practicing physical chemist who made contributions were a hallmark of his later work. After a couple of years, to thermodynamics, the determination of specific heats, and still in local government employment, he became a and to electrochemical theory. So, for example, he is not laboratory assistant in the town’s Pupil Teachers Train- mentioned in Keith Laidler’s World of Physical Chemis- ing College before finally becoming a clerk in Bolton’s try (1993). -

Lewis and Bronsted Concept of Acids and Bases
Lewis and Bronsted Concept of Acids and Bases Lewis Concept : Acids and bases are an important part of chemistry. One of the most applicable theories is the Lewis acid/base motif that extends the definition of an acid and base beyond H+ and OH- ions as described by Brønsted-Lowry acids and bases. Introduction The Brønsted acid-base theory has been used throughout the history of acid and base chemistry. However, this theory is very restrictive and focuses primarily on acids and bases acting as proton donors and acceptors. Sometimes conditions arise where the theory doesn't necessarily fit, such as in solids and gases. In 1923, G.N. Lewis from UC Berkeley proposed an alternate theory to describe acids and bases. His theory gave a generalized explanation of acids and bases based on structure and bonding. Through the use of the Lewis definition of acids and bases, chemists are now able to predict a wider variety of acid-base reactions. Lewis' theory used electrons instead of proton transfer and specifically stated that an acid is a species that accepts an electron pair while a base donates an electron pair. Figure 1 Above: A Lewis Base (B) donates it electrons to a Lewis Acid (A) resulting in a coordinate covalently bonded compound, also known as an adduct. The reaction of a Lewis acid and a Lewis base will produce a coordinate covalent bond, as shown in Figure 1 above. A coordinate covalent bond is just a type of covalent bond in which one reactant gives it electron pair to another reactant. -

Nature Vol 138-N3500.Indd
NovEMBER 28, 1936 NATURE 911 There are no doubt other examples where things, and to the importance of doing it carefully. improvements are needed, but the above are among It may also be hoped that the actual suggestions the most glaring. The present article does not aim may be of service, so that those future writers at inducing any exact conformity to its suggestions, who have not yet firmly established their own but rather at directing attention to the real diffi usages may be induced to accept at least some of culty in the invention of suitable names for new them. Control of the Prickly-pear in Australia HE control of the prickly-pears, Opuntia Since 1921, officers of the Board have visited T inermis and 0. stricta, in Australia affords most of the known prickly-pear regions of North one of the most outstanding examples of the and South America. Their investigations resulted application of biological knowledge to economic in the discovery of about 145 species of insects purpose. It needs to be recollected that in 1925, which appear to be confined, in feeding habits, to about sixty million acres of grazing and farming prickly-pears and other Cactacere. Fungal and land were known to be under infestation by bacterial diseases also came in for investigation, prickly-pear in Queensland and New South Wales: but it was revealed that they did not afford much the rate of spread of this scourge was stated to be promise of direct utility, since many of these reliably figured at almost one million acres a year. -

Hydronium Ion
Hydronium ion All acidic aqueous solutions contain protonated water, known commonly as the + hydronium ion (H3O ). Brønsted acids release one or more of their protons (hydrogen ions), which combine with water molecules. Lewis acids extract one or more hydroxyl ions from water to release hydrogen ions, which, again, form hydronium ions. So, when you see an aqueous chemical equation with the symbol H+, be assured that + the actual ionic species is H3O . Why is “oxonium” the preferred name for the hydronium ion? It’s because hydronium is the simplest form of oxonium ions, in which three entities are attached to an oxygen atom, resulting in a net positive charge. [In my opinion, hydronium is much more descriptive than oxonium.—Ed.] The concept of the hydronium ion has been known since the 19th century. In the 1880s, Swedish physicist/chemist Svante Arrhenius, working with German chemist Wilhelm Ostwald, defined an acid as a substance that dissociates in water to form hydrogen ions, which protonate water to form hydronium ions. Protonating acids became known as Arrhenius acids. Arrhenius worked at Sweden’s Royal Institute of Technology (Stockholm) for his entire career. Later in his career, he helped establish the Nobel Prizes. He won the award in chemistry in 1903 for his definitions of acids and bases. Not to be outdone, Ostwald won the 1909 Nobel Prize in Chemistry for his work in chemical reaction rates, equilibria, and catalysis. He worked at Riga Polytechnical Institute (Latvia) when he collaborated with Arrhenius. Arrhenius’s ideas were later refined, independently, by Johannes Brønsted at the University of Copenhagen and Martin Lowry at the University of Cambridge (UK). -
Acids & Bases Unit
Connected Chemistry Acids & Bases Unit Lesson 2: Brønsted-Lowry and Lewis Theories Student’s Lesson at a Glance Lesson Summary This lesson contains six activities that offer an extensive overview of the Brønsted-Lowry and Lewis theories of acids and bases. Following a Connecting Activity, teachers can demonstrate how to use a submicroscopic simulation to differentiate the theories. In this simulation, the user can switch between the Brønsted-Lowry view of an acid-base reaction and the Lewis view of the same reaction. Students continue the lesson by creating working definitions of these two theories and further explore other reactions via computer simulations to better understand the different acid- base perspectives. Students create detailed sketches and record observations. Students apply the theories to identify different substances. In the final activity, students complete a Venn diagram of the theories as well as a final review of the limitations of each model. SWBAT (Students Will Be Able To) • Compare and contrast the Arrhenius, Brønsted-Lowry, and Lewis theories of acids + + • Know that the Arrhenius theory states that acids generate H (H3O ) ions and bases generate OH– ions in water • Know that according to the Brønsted-Lowry theory of acids, acids donate protons and bases accept protons • Know that the Lewis acids are electron acceptors and bases are electron donors Essential Vocabulary Keep a list of all important words from this lesson. This list, in addition to the lists from other lessons, will make studying easier and improve scientific communication skills. The essential vocabulary from the unit is in bold. Additional words that will expand your scientific vocabulary are in italics. -

Sample Pages
A ACID ANHYDRIDES FIELDS OF STUDY anhydrides are also possible and are, in fact, essential components of certain biochemical cycles. Acid an- Organic Chemistry hydrides can also be thought of as esters formed from an acyl group. Generally, acid anhydrides are repre- SUMMARY sented as follows: O O O O The characteristic properties and reactions of acid | | | | | | | | anhydrides are discussed. Acid anhydrides are useful C C C C compounds in organic synthesis reactions, helping R O R Ar O Ar to form complex molecular structures from simple The generic placeholders R− and Ar− indicate an starting materials. alkyl group substituent and an aryl group substituent, respectively. PRINCIPAL TERMS Acid anhydrides of linear molecules are generally used to add single substituents to a molecular struc- acyl group: a functional group with the formula − ture. Acid anhydride compounds with a cyclic struc- RCO, where R is connected by a single bond to the ture are also commonly used in synthetic procedures carbon atom of the carbonyl (C=O) group. to elaborate a molecular structure. In principle, an carboxylic acid: an organic compound containing anhydride can be formed from any carboxylic acid. a carboxyl functional group and having the gen- However, in practice, very few acid anhydrides are eral formula RC(=O)OH. available, and other carboxylic-acid derivatives are functional group: a specifi c group of atoms with a more readily obtained for particular purposes. Thus, characteristic structure and corresponding chem- linear carboxylic acids are seldom prepared or uti- ical behavior within a molecule. lized. Dicarboxylic acids, in which the formation organic acid: an acid derived from an organic com- of the anhydride produces a fi ve- or six-membered pound. -

Acid-Base Concepts
Acids and Bases Overview Chemistry 362 MIT 3091 Video Lecture: Acids and Bases on You Tube http://ocw.mit.edu/courses/materials-science-and-engineering/3- 091sc-introduction-to-solid-state-chemistry-fall-2010/aqueous- solutions/26-acids-and-bases/ Acid-Base properties Focused on water and protons and hydroxide ions: Protic Acids: compounds that ionize to add to H+ ion concentration of Water. Bases: compounds that increase OH− concentration of water. Svante August Arrhenius 1859 – 1927 Arrhenius’ concept based on water Arrhenius, 1880s: + Acids form hydrogen ions H (H2O)n in aqueous solution. Bases form hydroxide ions in aqueous solution. Examples of Arrhenius acids (in water): HCl, H2SO4, etc. Examples of Arrhenius bases (in water): NaOH, NH3, etc. Arrhenius definitions only apply to aqueous solutions. A general Arrhenius acid-base reaction is the reaction between H+ and OH- to produce water. A Neutralization Reaction Acid + Base Salt + Water H+ + NO3- + K+ + OH- K+ + NO3- + H2O pH, pOH and other pBeasts In general, pX = -log10(X) pH = -log[H+] pOH = -log[OH-] pK = -logK In pure water, pH = pOH = 7 In acidic solution, pH <7; pOH > 7 In basic solutions, pH > 7, pOH < 7 Since pH + pOH = 14, either value is sufficient to describe both [H+] and [OH-] Johannes Nicolaus Brønsted Thomas Martin Lowry 1879 – 1947 1874 – 1936 Brønsted-Lowry Approach to Acids and Bases: Extends Arrhenius and Introduces Conjugate Acid/Base Pairs “fuzzy term” Brønsted and Lowry, 1923: Acid – a species with a capability to lose H+. Base – a species with a capability to gain H+. [As often as not Lowry’s name is omitted and only Brønsted’s name is used.] Brønsted’s acids and bases are by and large the same acids and bases as in the Arrhenius model but the model of Brønsted and Lowry is not restricted to aqueous solutions. -

Prof. TM Lowry, CBE
912 NATURE NOVEMBER 28, 1936 either been largely suppressed or their activities to other host plants cannot be neglected. In the nullified owing to competition with its larvre. It is case of prickly-pear control, elaborate biological only locally, and in relation to a few species of tests as to the host plant range and preferences of Opuntia of lesser importance, that the Cactoblastis such insects have been a feature of inestimable has proved more or less ineffective. Such problems, value. Doubtful species have been excluded and however, are being dealt with effectively through the none so far introduced has betrayed any tendency, operations of other phytophagous insects including other than of a sporadic nature, to resort to hosts cochineal (Dactylopius) and cerambycid beetles. outside the species of Opuntia. In any campaign involving the repression of pest We hope to refer to prickly-pear control again plants through the medium of introduced species at a later date when the promised book, re of insects, the potential danger that such insects, counting full details, becomes available. in a new environment, may transfer their activities A. D. lMMS. Obituary Prof. T. M. Lowry, C.B.E., F.R.S. hydroxylic solvent as cresol, or a ba;:;ic one like HOMAS MARTIN LOWRY, who died at pyridine, but proceeds almost too rapidly for measure T Cambridge on November 2, came of an old ment in a mixture of these solvents. Lowry thus Cornish family which had been long connected with showed that an amphoteric solvent is necessary as a the Methodist Church; he was born at Low Moor, catalyst for the mutarotation process, and up Bradford, Yorks, on October 26, 1874, the second his now well-known theory of prototropic change ; ;o;on of the Rev. -

Using History in Teaching Chemistry: History on Powerpoint
Using History in Teaching Chemistry: History on PowerPoint David A. Katz Department of Chemistry Pima Community College Tucson, AZ U.S.A. Voice: 520‐206‐6044 Email: [email protected] Web site: http://www.chymist.com Why History? • Except for the atomic theory and/or the periodic table, most textbooks do not include a historical background of the topics covered. • History puts faces on theories and concepts • Students find out where “all this stuff” comes from • A simplified theory aids in understanding as the concept becomes more complex Essays and Papers Relating to the History of Chemistry on www.chymist.com • This section on History of Chemistry and Science currently consists of five parts. • Parts 1 and 2 are essays and texts of historical interest Part 3 consists of photos and PowerPoint presentations of science related travels • Part 4 is a collection of PowerPoint presentations for use in teaching • Part 5 is some selected pictures of chemical art from my personal library. Some Items on Part 1 These are PDF files • An Illustrated History of Alchemy and Chemistry from ancient times to 1800 • The Splendor Solis A translation of the 1582 alchemical manuscript attributed to Solomon Trismosin with color photographs of 22 plates. • J. A. R. Newlands, The Periodic Law This is the complete text of Newlands' book, published in London in 1884, containing all of his published papers published from 1863 through 1878. Items on Part 4 • The Atomic Theory looks at the concepts of matter and atoms from ancient times to the structure of atoms. • The Periodic Classification tells the history of the development of the periodic table • Acids and Bases tells some history of acid‐base concepts and pH • Electrochemistry is a brief history of electricity and electrochemistry Items on Part 4 (continued) • Nuclear Chemistry tells the story of x‐rays and radioactivity through nuclear weapons • Thermodynamics relates some history of thermodynamics principles Items on Part 5 History on PowerPoint: Acids and Bases David A.