Sample Pages
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
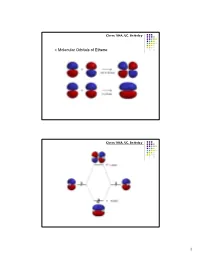
Π Molecular Orbitals of Ethene
Chem 104A, UC, Berkeley Molecular Orbitals of Ethene Chem 104A, UC, Berkeley 1 Chem 104A, UC, Berkeley Molecular Orbital Analysis of Ethene Dimerisation the reaction is said to be a "symmetry forbidden" – interestingly, this reaction is rare and very slow ! Chem 104A, UC, Berkeley Molecular Orbitals of 1,3-Butadiene 2 Chem 104A, UC, Berkeley Chem 104A, UC, Berkeley Molecular Orbital Analysis of Diels-Alder reaction 3 Chem 104A, UC, Berkeley Acid-Base Chemistry Reading : MT 6 Chem 104A, UC, Berkeley The Acid Base Theory of Brønsted and Lowry In 1923, within several months of each other, Johannes Nicolaus Brønsted (Denmark) and Thomas Martin Lowry (England) published essentially the same theory about how acids and bases behave. •An acid is a "proton donor." •A base is a "proton acceptor." 4 Chem 104A, UC, Berkeley + HCl + H2O <===> H3O + Cl¯ HCl - this is an acid, because it has a proton available to be transfered. H2O - this is a base, since it gets the proton that the acid lost. Now, here comes an interesting idea: + H3O - this is an acid, because it can give a proton. Cl¯ - this is a base, since it has the capacity to receive a proton. + Notice that each pair (HCl and Cl¯ as well as H2O and H3O differ by one proton (symbol = H+). These pairs are called conjugate pairs. Chem 104A, UC, Berkeley HCl + NaOH ---> H2O+ NaCl stronger stronger Weaker weaker conjugate conjugate acid base acid base + - NH4 +OH---> H2O+ NH3 stronger stronger weaker weaker conjugate conjugate acid base acid base 5 Chem 104A, UC, Berkeley H n A H 2O H n1 A H3O [H n1 A ][H3O ] pKa1 log [H n A] pKa 0 strong pKa 0 weak Chem 104A, UC, Berkeley Amphoteric Compound H n A H 2O H n1 A H3O NH 3 H 2O NH 4 OH 6 Chem 104A, UC, Berkeley Oxyacids AOp(OH)q A=Si, N, P, As, S, Se, Te, Cl, Br, I p=# of nonhydrogenated oxygen atom H + A ----O As atom A is rendered more positive, it becomes easier to break the O-H bond Because of enhanced bond polarization. -

EPA Handbook: Optical and Remote Sensing for Measurement and Monitoring of Emissions Flux of Gases and Particulate Matter
EPA Handbook: Optical and Remote Sensing for Measurement and Monitoring of Emissions Flux of Gases and Particulate Matter EPA 454/B-18-008 August 2018 EPA Handbook: Optical and Remote Sensing for Measurement and Monitoring of Emissions Flux of Gases and Particulate Matter U.S. Environmental Protection Agency Office of Air Quality Planning and Standards Air Quality Assessment Division Research Triangle Park, NC EPA Handbook: Optical and Remote Sensing for Measurement and Monitoring of Emissions Flux of Gases and Particulate Matter 9/1/2018 Informational Document This informational document describes the emerging technologies that can measure and/or identify pollutants using state of the science techniques Forward Optical Remote Sensing (ORS) technologies have been available since the late 1980s. In the early days of this technology, there were many who saw the potential of these new instruments for environmental measurements and how this technology could be integrated into emissions and ambient air monitoring for the measurement of flux. However, the monitoring community did not embrace ORS as quickly as anticipated. Several factors contributing to delayed ORS use were: • Cost: The cost of these instruments made it prohibitive to purchase, operate and maintain. • Utility: Since these instruments were perceived as “black boxes.” Many instrument specialists were wary of how they worked and how the instruments generated the values. • Ease of use: Many of the early instruments required a well-trained spectroscopist who would have to spend a large amount of time to setup, operate, collect, validate and verify the data. • Data Utilization: Results from path integrated units were different from point source data which presented challenges for data use and interpretation. -

Preparation and Purification of Atmospherically Relevant Α
Atmos. Chem. Phys., 20, 4241–4254, 2020 https://doi.org/10.5194/acp-20-4241-2020 © Author(s) 2020. This work is distributed under the Creative Commons Attribution 4.0 License. Technical note: Preparation and purification of atmospherically relevant α-hydroxynitrate esters of monoterpenes Elena Ali McKnight, Nicole P. Kretekos, Demi Owusu, and Rebecca Lyn LaLonde Chemistry Department, Reed College, Portland, OR 97202, USA Correspondence: Rebecca Lyn LaLonde ([email protected]) Received: 31 July 2019 – Discussion started: 6 August 2019 Revised: 8 December 2019 – Accepted: 20 January 2020 – Published: 9 April 2020 Abstract. Organic nitrate esters are key products of terpene oxidation in the atmosphere. We report here the preparation and purification of nine nitrate esters derived from (C)-3- carene, limonene, α-pinene, β-pinene and perillic alcohol. The availability of these compounds will enable detailed investigations into the structure–reactivity relationships of aerosol formation and processing and will allow individual investigations into aqueous-phase reactions of organic nitrate esters. Figure 1. Two hydroxynitrate esters with available spectral data. Relative stereochemistry is undefined. 1 Introduction derived ON is difficult, particularly due to partitioning into the aerosol phase in which hydrolysis and other reactivity Biogenic volatile organic compound (BVOC) emissions ac- can occur (Bleier and Elrod, 2013; Rindelaub et al., 2014, count for ∼ 88 % of non-methane VOC emissions. Of the to- 2015; Romonosky et al., 2015; Thomas et al., 2016). Hydrol- tal BVOC estimated by the Model of Emission of Gases and ysis reactions of nitrate esters of isoprene have been stud- Aerosols from Nature version 2.1 (MEGAN2.1), isoprene is ied directly (Jacobs et al., 2014) and the hydrolysis of ON estimated to comprise half, and methanol, ethanol, acetalde- has been studied in bulk (Baker and Easty, 1950). -

Volatile Organic Compound Production in Synechococcus WH8102
Volatile Organic Compound Production in Synechococcus WH8102 by Duncan Ocel A THESIS submitted to Oregon State University Honors College in partial fulfillment of the requirements for the degree of Honors Baccalaureate of Science in Chemistry and Botany (Honors Scholar) Presented May 21, 2018 Commencement June 2018 2 3 AN ABSTRACT OF THE THESIS OF Duncan Ocel for the degree of Honors Baccalaureate of Science in Chemistry and Botany presented on May 21, 2018. Title: Volatile Organic Compound Production in Synechococcus WH8102. Abstract approved:_____________________________________________________ Kimberly Halsey High-resolution mass spectrometry was used to measure a range of volatile organic compounds (VOCs) in real time as they were produced by the ubiquitous marine cyanobacterium Synechococcus WH8102 during a 24-hour light/dark cycle. Ethenone, acetaldehyde, ethanol, isoprene, acetic acid, dimethyl sulfide (DMS), acetone, phenol, and several as-yet unidentified compounds were measured in higher concentration in live cultures than in azide-killed cultures or sterile artificial seawater. Several compounds were found in higher concentration in the daylight part of the diel cycle than in the night, suggesting VOCs are produced during active photosynthesis. Key Words: phytoplankton, volatile organic compounds, Synechococcus, acetaldehyde, dimethyl sulfide Corresponding e-mail address: [email protected] 4 ©Copyright by Duncan Ocel May 21,2018 All Rights Reserved 5 Volatile Organic Compound Production in Synechococcus WH8102 by Duncan Ocel A THESIS submitted to Oregon State University Honors College in partial fulfillment of the requirements for the degree of Honors Baccalaureate of Science in Chemistry and Botany (Honors Scholar) Presented May 21, 2018 Commencement June 2018 6 Honors Baccalaureate of Science in Chemistry and Botany project of Duncan Ocel presented on May 21, 2018. -

Notes Acids and Bases
Notes Acids and Bases Arrhenius Acid Base Theory: In 1884 Svante Arrhenius proposed the first theoretical model for acids and bases. Prior to that time, these chemically opposite substances were described in properties such as their taste; their effects on metals, carbonates, and dyes (called indicators); their feel to the touch, and their ability to react with each other. According to the Arrhenius theory , pure water dissociates to some extent to produce hydrogen ions, H + and hydroxide ions, OH -. When this occurs, equal amounts of H + and OH - ions are produced: + - H2O(l) H (aq) + OH (aq) An acid, according to Arrhenius, is any substance that liberates H + ions when placed in water. When the H + concentration is elevated the OH - concentration decreases, this solution is said to be acidic. Similarly, a base is defined as any substance that liberates OH - ions when placed in water. The resulting solution has a higher concentration of OH - ions than H + ions and is said to be basic, or alkaline. + - ACID: HCl (aq) H (aq) + Cl (aq) + - BASE: NaOH (aq) Na (aq) + OH (aq) Brønsted - Lowry Acid Base Theory: A more general and realistic theory was proposed by two chemists working independent of the other, Johannes Nicolaus Brønsted of Denmark and Thomas Martin Lowry of England. Both chemists are given credit; the theory is named the Brønsted-Lowry theory . Acids are defined as substances that donate H + ions, protons and therefore are called proton donators, while bases are substances that accept protons and are defined as proton acceptors. On this basis, the autoionization of water is given as follows: + - H2O(l) + H 2O(l) H3O (aq) + OH (aq) In this reaction, one water molecule donates a proton, H +, and behaves as an acid, while the other water molecule accepts the proton and behaves as a base. -

Oxidation of N-Butylcyclohexane in the Low Temperature Region
CORE Metadata, citation and similar papers at core.ac.uk Provided by Drexel Libraries E-Repository and Archives Oxidation of n-Butylcyclohexane in the Low Temperature Region A Thesis Submitted to the Faculty of Drexel University by Robert Harris Natelson in partial fulfillment of the requirements for the degree of Doctor of Philosophy May 2010 © Copyright 2010 Robert H. Natelson. All Rights Reserved. ii DEDICATIONS This work is dedicated to my mom and dad. iii ACKNOWLEDGMENTS I would first like to thank my advisors Dr. Nicholas P. Cernansky and Dr. David L. Miller for providing me the opportunity to conduct my graduate research studies under their guidance. Our lively discussions have been an important part of my learning process. I would also like to express my gratitude to my Ph.D. Defense committee, including Drs. Cernansky, Miller, Howard Pearlman, Ying Sun, and James Tangorra for their time, questions, and suggestions. I would also like to thank Dr. Vedha Nayagam at NASA Glenn Research Center for first introducing me to the research area of combustion. My colleague at Hess Lab, Matthew Kurman, has been an invaluable collaborator in my work, and I cannot imagine completing this study without his support in the laboratory. I would also like to acknowledge the thoughtful discussions I have had with my other Hess Lab friends, including Jamie Lane, Ashutosh Gupta, Rodney Johnson, David Lenhert, Xiaohui Gong, Jincai Zheng, Seuk Chun Choi, Yi Ma, Laton McGibbon, Kevin Wujcik, Brian Folkes, Haider Hasan, Farinaz Farid, and Julius Corrubia. The MEM department and Hess Lab staff, including Ms. -

Preparation and Purification of Atmospherically Relevant Α
Technical Note: Preparation and purification of atmospherically relevant a-hydroxynitrate esters of monoterpenes Elena Ali McKnight, Nicole P. Kretekos, Demi Owusu, Rebecca Lyn LaLonde 5 Chemistry Department, Reed College, Portland, OR, 97202, USA Correspondence to: Rebecca Lyn LaLonde ([email protected]) Abstract. Organic nitrate esters are key products of terpene oxidation in the atmosphere. We report here the preparation and purification of nine nitrate esters derived from (+)-3-carene, limonene, a-pinene, b-pinene and perillic alcohol. The availability of these compounds will enable detailed investigations into the structure 10 reactivity relationships of aerosol formation and processing and will allow individual investigations into aqueous phase reactions of organic nitrate esters. 1 Introduction Biogenic volatile organic compound (BVOC) emissions account for ~88% of non-methane VOC emissions. Of the total BVOC estimated by the Model of Emission of Gases and Aerosols from Nature version 2.1 15 (MEGAN2.1), isoprene is estimated to comprise half, and methanol, ethanol, acetaldehyde, acetone, a-pinene, b-pinene, limonene, ethene and propene together encompass another 30%. Of the terpenoids, a-pinene alone is estimated to generate ~66 Tg year -1 (Guenther et al., 2012). These monoterpenes can be oxidized by nitrate radicals that are projected to account for more than half of the monoterpene-derived secondary organic aerosol (SOA) in the US (Pye et al., 2010). Nitrate oxidation pathways have been shown to be important particularly 20 during nighttime. A large portion (30-40%) of monoterpene emissions occur at night (Pye et al., 2010). These emissions can then react with NO3 radicals, formed from the oxidation of NO2 emissions by O3, (Pye et al., 2010). -

Article Is Available On- Line At
Atmos. Chem. Phys., 21, 11505–11518, 2021 https://doi.org/10.5194/acp-21-11505-2021 © Author(s) 2021. This work is distributed under the Creative Commons Attribution 4.0 License. Unexplored volatile organic compound emitted from petrochemical facilities: implications for ozone production and atmospheric chemistry Chinmoy Sarkar1,a, Gracie Wong1, Anne Mielnik1, Sanjeevi Nagalingam1, Nicole Jenna Gross1, Alex B. Guenther1, Taehyoung Lee2, Taehyun Park2, Jihee Ban2, Seokwon Kang2, Jin-Soo Park3, Joonyoung Ahn3, Danbi Kim3, Hyunjae Kim3, Jinsoo Choi3, Beom-Keun Seo4, Jong-Ho Kim4, Jeong-Ho Kim5, Soo Bog Park4, and Saewung Kim1 1Department of Earth System Science, University of California Irvine, Irvine, CA 92697, USA 2Department of Environmental Science, Hankuk University of Foreign Studies, Yongin 17035, South Korea 3Air Quality Research Division, National Institute of Environmental Research, Incheon 22689, South Korea 4Institute of Environmental Research, Hanseo University, Seosan-si, South Korea 5APM Engineering Co. Ltd., Bucheon-si, South Korea anow at: Air Quality Research Center, University of California Davis, Shields Avenue, Davis, CA 95616, USA Correspondence: Chinmoy Sarkar ([email protected]), Jin-Soo Park ([email protected]), and Saewung Kim ([email protected]) Received: 22 October 2020 – Discussion started: 2 November 2020 Revised: 6 July 2021 – Accepted: 6 July 2021 – Published: 2 August 2021 Abstract. A compound was observed using airborne pro- potential to significantly influence local photochemistry, and ton transfer reaction time-of-flight mass spectrometry (PTR- therefore, further studies focusing on the photooxidation and TOF-MS) measurements in the emission plumes from the atmospheric fate of ketene through chamber studies are re- Daesan petrochemical facility in South Korea. -

PDF (Detailed Experimental Procedures, Full
CO Coupling Chemistry of a Terminal Mo Carbide: Sequential Addition of Proton, Hydride, and CO Releases Ethenone Joshua A. Buss, Gwendolyn A. Bailey, Julius Oppenheim, David G. VanderVelde, William A. Goddard III, and Theodor Agapie Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Blvd. MC 127-72, Pasadena, CA, USA Supporting Information Contents Experimental Details S3 General Considerations S3 Carbide–CO Coupling from Carbide 1 S3 In situ generation of carbide 1 S3 Figure S1— Partial 13C{1H} (126 MHz, THF, -50 °C) and 31P{1H} (202 MHz, THF, -50 °C) NMR spectra showing clean deprotonation of methylidyne 4 with benzyl potassium. S4 Addition of CO to Carbide 1 to form Metallaketene Complex 2 S4 1 Figure S2— H NMR Spectrum (400 MHz, C6D6, 23 °C) of 2. S5 31 1 Figure S3— P{ H} NMR Spectrum (162 MHz, C6D6, 23 °C) of 2. S5 13 1 Figure S4— C{ H} NMR Spectrum (101 MHz, C6D6, 23 °C) of 2. S6 Figure S5—31P{1H} NMR spectrum (202 MHz, THF, 23 °C) of 2-13C. S6 Figure S6—13C{1H} NMR spectrum (126 MHz, THF, 23 °C) of 2-13C. S6 Preparation of a mixture predominantly of carbide isomer 3 S7 1 13 Figure S7— H NMR Spectrum (400 MHz, C6D6, 23 °C) of 3- C. S8 31 1 13 Figure S8— P{ H} NMR spectrum (162 MHz, C6D6, 23 °C) of 3- C. S8 13 1 13 Figure S9— C{ H} NMR spectrum (101 MHz, C6D6, 23 °C) of 3- C. S8 Sequential Addition of Proton, Hydride, and CO to Carbide 1 S9 Synthesis of Methylidyne 4 S9 1 Figure S10— H NMR Spectrum (300 MHz, C6D6, 23 °C) of 4. -

Compilation of Chemical Kinetic Data for Combustion Chemistry. Part 2. Non-Aromatic C, H, O, N, and S Containing Compounds
v NBS PUBLICATIONS r A111D2 TBOmi NAT'L INST OF STANDARDS & TECH R.I.C. All 102730091 Westley, Francls/Compllatlon of chemical 2 QC100 .11573 NO. 73 V2;1987 C.2 NBS-PUB-C NSRDS-NBS 73, Part U.S. DEPARTMENT OF COMMERCE / National Bureau of Standards 1 BB he National Bureau of Standards was established by an act of Congress on March 3, 1901. The Bureau’s overall M goal is to strengthen and advance the nation’s science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research to assure international competidveness and leadership of U.S. industry, science arid technology. NBS work involves development and transfer of measurements, standards and related science and technology, in support of continually improving U.S. productivity, product quality and reliability, innovation and underlying science and engineering. The Bureau’s technical work is performed by the National Measurement Laboratory, the National Engineering Laboratory, the Institute for Computer Sciences and Technology, and the Institute for Materials Science and Engineering. The National Measurement Laboratory Provides the national system of physical and chemical measurement; • Basic Standards 2 coordinates the system with measurement systems of other nations and • Radiation Research furnishes essential services leading to accurate and uniform physical and • Chemical Physics chemical measurement throughout the Nation’s scientific community, • Analytical Chemistry industry, and commerce; provides advisory and research -

History and the Teaching of Chemistry. a Tribute to Thomas Lowry's Textbook
Educación Química (2016) 27, 175---181 educación Química www.educacionquimica.info REFLECTION History and the teaching of chemistry. A tribute to Thomas Lowry’s textbook ‘‘Historical Introduction to Chemistry’’ William B. Jensen Department of Chemistry, University of Cincinnati, Cincinnati, OH 45221-0172, United States Received 9 March 2016; accepted 30 March 2016 Available online 11 June 2016 KEYWORDS Abstract Through Thomas Lowry’s book written in 1915, Historical Introduction to Chemistry, Words history this paper shows how chemistry can be taught from its own history. of chemistry; All Rights Reserved © 2016 Universidad Nacional Autónoma de México, Facultad de Química. Chemistry teaching; This is an open access item distributed under the Creative Commons CC License BY-NC-ND 4.0. Historical approach PALABRAS CLAVE Historia y Ensenanza˜ de la Química. Un tributo al libro de Thomas Lowry Historia de la ‘‘Introducción Histórica a la Química’’ química; Resumen A través del libro de Thomas Lowry escrito en 1915, Historical Introduction to Chem- Ensenanza˜ de la química; istry, este artículo muestra la manera de cómo se puede ensenar˜ química a partir de su propia Aproximación historia. histórica Derechos Reservados © 2016 Universidad Nacional Autónoma de México, Facultad de Química. Este es un artículo de acceso abierto distribuido bajo los términos de la Licencia Creative Commons CC BY-NC-ND 4.0. Though much has been written advocating the use of his- introductory textbooks which claim to include some his- tory of chemistry in the teaching of introductory chemistry tory of chemistry, usually in the form of portraits of select courses at both the high school and college levels, there chemists from the past with short biographical captions have been surprisingly few attempts to write a proper text- attached or, in a few cases, supplementary boxes briefly book based on this approach. -

Optical Gas Imaging
OPTICAL GAS IMAGING Infrared Cameras for Gas Leak Detection MAKE INVISIBLE GASES VISIBLE SAVE LIVES, REVENUE, AND THE DAY A facility can have thousands of connections and fittings that require regular inspection, but the reality is only a small percentage of these components will ever leak. Testing them all with a traditional “sniffer” takes a great deal of time, effort and may put the inspector in an unsafe environment. SEE HYDROCARBON LEAKS CLEARLY FIND SF6 LEAKS EASILY FIND LEAKS AT STEEL PLANTS METHANE AND HYDROCARBONS SULFUR HEXAFLUORIDE (SF6) CARBON MONOXIDE (CO) Scan thousands of connections for natural gas Scan substation circuit breakers for sulfur hexafluoride Protect workers and the environment from toxic levels (methane) and other hydrocarbon leaks quickly and from (SF6) leaks at a safe distance from high-voltage areas, of carbon monoxide (CO) by pinpointing leaks quickly a safe distance to avoid regulatory violations, fines, and without the need to shut down operations. and efficiently. lost revenue. Optical gas imaging cameras give you the power to spot invisible gases as they escape, so you can find fugitive emissions faster and more reliably than with sniffer detectors. With a FLIR GF-Series camera, you can document gas leaks that lead to lost product, lost revenue, fines, and safety hazards. DETECT LEAKS FROM HYDROGEN-COOLED GENERATORS SPOT HARD-TO-FIND CO2 LEAKS DETECT R-124 COMPRESSOR LEAKS HYDROGEN (CO TRACER GAS) CARBON DIOXIDE (CO ) REFRIGERANTS From natural gas extraction to 2 2 petrochemical operations and power Imaging the tracer gas, CO2, with an optical gas camera Prevent shut-downs by detecting carbon dioxide Find leaks early to avoid interruptions in operations, generation, companies have saved allows operators of hydrogen-cooled generators to (CO2) leaks early in chemical production, manufacturing, and prevent the loss of perishable products, and limit the more than $10 million annually in lost efficiently find hydrogen leaks.