J. R. Partington (1886-1965): Physical Chemistry in Deed and Word
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Geoffrey Wilkinson
THE LONG SEARCH FOR STABLE TRANSITION METAL ALKYLS Nobel Lecture, December 11, 1973 by G EOFFREY W ILKINSON Imperial College of Science & Technology, London, England Chemical compounds in which there is a single bond between a saturated car- bon atom and a transition metal atom are of unusual importance. Quite aside from the significance and role in Nature of the cobalt to carbon bonds in the vitamin B 12 system and possible metal to carbon bonds in other biological systems, we need only consider that during the time taken to deliver this lec- ture, many thousands, if not tens of thousands of tons of chemical compounds are being transformed or synthesised industrially in processes which at some stage involve a transition metal to carbon bond. The nonchemist will pro- bably be most familiar with polyethylene or polypropylene in the form of do- mestic utensils, packaging materials, children’s toys and so on. These materials are made by Ziegler-Natta* or Philipps’ catalysis using titanium and chro- mium respectively. However, transition metal compounds are used as catalysts in the synthesis of synthetic rubbers and other polymers, and of a variety of simple compounds used as industrial solvents or intermediates. For example alcohols are made from olefins, carbon monoxide and hydrogen by use of cobalt or rhodium catalysts, acetic acid is made by carbonylation of methanol using rhodium catalysts and acrylonitrile is dimerised to adiponitrile (for nylon) by nickel catalysts. We should also not forget that the huge quantities of petroleum hydrocarbons processed by the oil and petrochemical industry are re-formed over platinum, platinum-rhenium or platinum-germanium sup- ported on alumina. -
Π Molecular Orbitals of Ethene
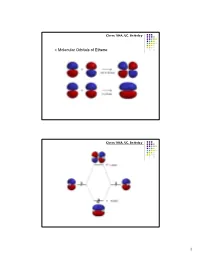
Chem 104A, UC, Berkeley Molecular Orbitals of Ethene Chem 104A, UC, Berkeley 1 Chem 104A, UC, Berkeley Molecular Orbital Analysis of Ethene Dimerisation the reaction is said to be a "symmetry forbidden" – interestingly, this reaction is rare and very slow ! Chem 104A, UC, Berkeley Molecular Orbitals of 1,3-Butadiene 2 Chem 104A, UC, Berkeley Chem 104A, UC, Berkeley Molecular Orbital Analysis of Diels-Alder reaction 3 Chem 104A, UC, Berkeley Acid-Base Chemistry Reading : MT 6 Chem 104A, UC, Berkeley The Acid Base Theory of Brønsted and Lowry In 1923, within several months of each other, Johannes Nicolaus Brønsted (Denmark) and Thomas Martin Lowry (England) published essentially the same theory about how acids and bases behave. •An acid is a "proton donor." •A base is a "proton acceptor." 4 Chem 104A, UC, Berkeley + HCl + H2O <===> H3O + Cl¯ HCl - this is an acid, because it has a proton available to be transfered. H2O - this is a base, since it gets the proton that the acid lost. Now, here comes an interesting idea: + H3O - this is an acid, because it can give a proton. Cl¯ - this is a base, since it has the capacity to receive a proton. + Notice that each pair (HCl and Cl¯ as well as H2O and H3O differ by one proton (symbol = H+). These pairs are called conjugate pairs. Chem 104A, UC, Berkeley HCl + NaOH ---> H2O+ NaCl stronger stronger Weaker weaker conjugate conjugate acid base acid base + - NH4 +OH---> H2O+ NH3 stronger stronger weaker weaker conjugate conjugate acid base acid base 5 Chem 104A, UC, Berkeley H n A H 2O H n1 A H3O [H n1 A ][H3O ] pKa1 log [H n A] pKa 0 strong pKa 0 weak Chem 104A, UC, Berkeley Amphoteric Compound H n A H 2O H n1 A H3O NH 3 H 2O NH 4 OH 6 Chem 104A, UC, Berkeley Oxyacids AOp(OH)q A=Si, N, P, As, S, Se, Te, Cl, Br, I p=# of nonhydrogenated oxygen atom H + A ----O As atom A is rendered more positive, it becomes easier to break the O-H bond Because of enhanced bond polarization. -

14-1676 Number One First Street
Getting to Number One First Street St Peter’s Square Metrolink Stop T Northbound trams towards Manchester city centre, T S E E K R IL T Ashton-under-Lyne, Bury, Oldham and Rochdale S M Y O R K E Southbound trams towardsL Altrincham, East Didsbury, by public transport T D L E I A E S ST R T J M R T Eccles, Wythenshawe and Manchester Airport O E S R H E L A N T L G D A A Connections may be required P L T E O N N A Y L E S L T for further information visit www.tfgm.com S N R T E BO S O W S T E P E L T R M Additional bus services to destinations Deansgate-Castle field Metrolink Stop T A E T M N I W UL E E R N S BER E E E RY C G N THE AVENUE ST N C R T REE St Mary's N T N T TO T E O S throughout Greater Manchester are A Q A R E E S T P Post RC A K C G W Piccadilly Plaza M S 188 The W C U L E A I S Eastbound trams towards Manchester city centre, G B R N E R RA C N PARKER ST P A Manchester S ZE Office Church N D O C T T NN N I E available from Piccadilly Gardens U E O A Y H P R Y E SE E N O S College R N D T S I T WH N R S C E Ashton-under-Lyne, Bury, Oldham and Rochdale Y P T EP S A STR P U K T T S PEAK EET R Portico Library S C ET E E O E S T ONLY I F Alighting A R T HARDMAN QU LINCOLN SQ N & Gallery A ST R E D EE S Mercure D R ID N C SB T D Y stop only A E E WestboundS trams SQUAREtowards Altrincham, East Didsbury, STR R M EN Premier T EET E Oxford S Road Station E Hotel N T A R I L T E R HARD T E H O T L A MAN S E S T T NationalS ExpressT and otherA coach servicesO AT S Inn A T TRE WD ALBERT R B L G ET R S S H E T E L T Worsley – Eccles – -

The Warburtons of Hale Barns
The Warburtons of Hale Barns Last Updated 5th September 2021 ©2018, 2019, 2020, 2021 Ray Warburton PREFACE This is my own tree. It originated in Hale Barns and was well established by 1600. My earliest certain ancestor is George (died 1639), but there is evidence his father was Thomas (died 1634). The tree is shown in several charts to make them a manageable size.The Ringley Clan is linked by DNA and is probably linked genealogically to the Mobberley branch. Table of Contents Hale Barns Preface i Surnames 1 Descendants of Thomas Warburton & Alice First Generation 3 Second Generation 5 Third Generation 8 Fourth Generation 11 Fifth Generation 18 Sixth Generation 30 Seventh Generation 44 Eighth Generation 61 Ninth Generation 95 Tenth Generation 140 Eleventh Generation 178 Twelfth Generation 206 Place Index 214 Person Index 251 ii Surnames A Artingstall, Ashley, Ashworth, Atkinson B Bailey, Bancks, Bancroft, Barber, Barlow, Barnett, Barrington, Barrow, Batty, Bayley, Beech, Bennet, Bennett, Bentley, Benton, Beswick, Bibby, Birch, Blackhurst, Blackshaw, Bleakly, Blomeley, Blows, Boon, Booth, Bourne, Bowers, Bracegirdle, Braddock, Bradshaw, Bray, Brereton, Brocklehurst, Brook, Broughton, Burden, Burgess, Burrows, Burton C Carter, Cartwright, Castalaneli, Cheetham, Cherry, Clarke, Clements, Cliff, Cliffe, Coan, Colclough, Colley, Collis, Consterdine, Cooke, Cooper, Coppock, Coxon, Cragg, Cresswell, Crosby, Cross, Crowe D Dalenoord, Darbyshire, Darlington, Davenport, Davies, Dean, Deardon, Debenham, Devis, Dicken, Dickin, Dooley, Durber, Dutton -

Bollin Valley Way: 5. Time Travel
Dainewell Woods Carrington Walks & Cycle Routes Works Moss Glazebrook Cadishead Sinderland Brook Bollin Valley Way Glaze & Footpath Junctions Brook 245* B5212 Trans Pennine Trail (NCN 62) 247 Cheshire Ring Canal Walk A57 Broadheath Dunham Circular Cycle Ride Sinderland 247 N. Tatton Cycle Trail 'A' PARTINGTON Green Dairy- house Bridleway P R T i 245 A6144 Farm * Permissive Bridleway Redbrook Crematorium Higher House Other Public Footpaths 247 247 Permissive Footpaths Hollins P R Green T Red House Farm P KEY Mosshall Black Moss Farm Farm 13 Roads Peterhouse Oldfield Brow 1ml Fences/Hedges B5159 Farm Bridgewater 1.6km Canals / Rivers Canal 13 Altrincham Toll B M Lakes / Reservoirs Bridge A57 5 B5160 National Trust Properties 38 38 P Golf Course Railway Mossbrow Higher Carr N Warburton Green Farm Built-up Area 5 Buildings Manchester Dunham Town Ship Canal 38 Forest / Woodland Dunham Woodhouses 38 B5160 Bowdon B5160 Picnic Site 37, 37A River Bollin , P Parking Dunham 38, 289 Aqueduct Dunham Park Country Pub Bollin P R T i R Other Refreshment Point T Toilets Heatley 37, 37A, 289 i Information Little Train Station B5159 Bollington B5161 A56 M Metro Link A6144 B Bus Station 5 New Farm 38 38 Bus Services Agden 37, 37A See overleaf for details Bridge A56 P R T i 1 km Access Involves Steps LYMM P © Crown copyright. All rights reserved. 289 M56 1 mile Cheshire County Council Licence No.100019582.2004 A56 Tatton Park 5mls. A556 Jn 8 The Bollin Valley Way and other recreational routes between Bowdon and Partington, including Dunham Massey. they are reasonably level – please ring and check. -

Stoddart Research Professor of Chemistry, a Position Northwestern University He Held Until His Death in 1977
PREVIOUS J.K.N. JONES JONES LECTURERS John Kenyon Netherton Jones, Ph.D. Department of Chemistry Birmingham University. Assistant lecturer Queen’s University and then lecturer at Bristol University 1936- 2011 • J.A. Caruso 1944, he was engaged in munitions research and training during the Second World War. 2010 • T. Marks He resigned at the end of the war with the is honoured to host the rank of captain, and returned to academic 2012 Jones Lecturer: work as senior lecturer at Manchester 2010 • G. van Koten University 1945-1948 and then as reader in chemistry at Bristol University 1948-1953. 2009 • P.B. Corkum He came to Queen's in 1953 as Chown Prof. J Fraser Stoddart Research Professor of Chemistry, a position Northwestern University he held until his death in 1977. 2008 • M. Gruebele Evanston, Illinois, USA Professor Jones' outstanding achievements in carbohydrate chemistry were recognized by 2005 • W. Klemperer his election as Fellow of the Royal Society of London in 1957 and of the Royal Society of Canada in 1959. The Division of 2001 • G. Ozin Carbohydrate Chemistry of the American Chemical Society presented him with the 1997 • M.S. Brookhart Claude S. Hudson Award in 1969, and in 1975 he received the Anselme Payen Award from the Cellulose, Paper and Textile 1993 • B.O. Fraser-Reid Public Lecture Division. In March 1975 he was awarded the third Sir Norman Haworth Memorial Medal of “Mingling Art with Science” The Chemical Society (London). 1990 • S. Hanessian Professor Jones was, at all times, an Thursday, March 22, 2012 educator of the highest rank and an 1982 • R. -

Vitamin C: Prevention of Chronic Diseases and Optimal Doses
NFT 9-2010 s. 20-27 08.09.10 13.00 Side 20 Vitenskap Review Vitamin C: Prevention of Chronic Diseases and Optimal Doses TEXT: Purusotam Basnet, Department of Pharmacy, Faculty of Health Sciences, University of Tromsø, e-mail: [email protected] INTRODUCTION acid because of its anti-scorbutic property ABSTRACT It has been known for a long time that (Latin word scorbutus = scurvy). In absence of fresh fruit and vegetables in the solution, it releases a proton to give an The role of vitamin C in prevention and human diet leads to scurvy, a fatal disease anion called ascorbate. Ascorbate easily treatment of scurvy is well accepted. In widely described throughout written transfers one electron and one additional spite of having long history as the candidate of alternative therapy for the history (Lind, 1753). Later, it was dis - proton and can remains in the stable prevention and treatment of cancer, still covered that ascorbic acid in fresh fruits radical form as semidehydroascorbate there is no common conclusion on the and vegetables prevents scurvy and that is (SDA), a state in between dehydroa- topic. However, its biochemical reaction why it is a vitamin for humans and must be scorbic acid (DHA) and ascorbate in the as an antioxidant and its immuno- a part of human diet (Mandl et al., 2009). physiological condition (figure 1). stimulating effects drew further Among the long list of people who Because of the unique electron trans- attention towards its health beneficial contributed to the knowledge regarding ferring capability, ascorbate plays a vital effects. Current official recommended vitamin C, three Nobel prize-winners are in role in living cells. -

Ian Rae: “Two Croatian Chemists Who Were Awarded the Nobel Prize in Chemistry”
Croatian Studies Review 13 (2017) Ian Rae: “Two Croatian Chemists who were Awarded the Nobel Prize in Chemistry” Ian Rae School of Chemistry University of Melbourne [email protected] Abstract Two organic chemists of Croatian origin, Leopold Ružička and Vladimir Prelog, made significant contributions to natural product chemistry during the twentieth century. They received their university education and research training in Germany and Czechoslovakia, respectively. Both made their careers in Zürich, Switzerland, and both shared the Nobel Prize in Chemistry, in 1939 and 1975, respectively. In this article, I have set the details of their lives and achievements against the education and research climates in Europe and other regions, especially as they apply to the field of chemistry. Key words: Croatia, organic, chemistry, Nobel, Ružička, Prelog 31 Croatian Studies Review 13 (2017) Introduction1 In the twentieth century two organic chemists of Croatian origin were awarded the Nobel Prize in Chemistry. They were Lavoslav (Leopold) Ružička (1887-1976) and Vladimir Prelog (1906-1998), whose awards came in 1939 and 1975, respectively. Both were living and working in Switzerland at the time of the awards and it was in that country – specifically in the city of Zürich – that they performed the research that made them Nobel Laureates. To understand the careers of Ružička and Prelog, and of many other twentieth century organic chemists, we need to look back to the nineteenth century when German chemists were the leaders in this field of science. Two developments characterise this German hegemony: the introduction of the research degree of Doctor of Philosophy (PhD), and the close collaboration between organic chemists in industry and university. -

Materials Science
Materials Science Materials Science is an interdisciplinary field combining physics (fundamental laws of nature), chemistry (interactions of atoms) and biology (how life interacts with materials) to elucidate the inherent properties of basic and complex systems. This includes optical (interaction with light), electrical (interaction with charge), magnetic and structural properties of everyday electronics, clothing and architecture. The Materials Science central dogma follows the sequence: Structure—Properties—Design—Performance. This involves relating the nanostructure of a material to its macroscale physical and chemical properties. By understanding and then changing the structure, material scientists can create custom materials with unique properties. The goal of the materials science minor is to create a cross-disciplinary approach to fundamental topics in basic and applied physical sciences. Students will gain experience and perspectives from the disciplines of chemistry, physics and biology. The minor places a strong emphasis on current nanoscale research methods in addition to the basics of electronic, optical and mechanical properties of materials. Any student with an interest in pursuing the cross-disciplinary minor in materials science should consult with the coordinator of the minor. Students are encouraged to declare their participation in their sophomore year but no later than the end of the junior year. Students also should seek an adviser from participating faculty. Degree Requirements for the Minor General College requirements -

Streamlining Alloy Design and Thermo-Mechanical Processing Parameters for High Strength Line Pipe Steels and Hot Rolled Microall
University of Texas at El Paso DigitalCommons@UTEP Open Access Theses & Dissertations 2018-01-01 Streamlining Alloy Design And Thermo- Mechanical Processing Parameters For High Strength Line Pipe Steels And Hot Rolled Microalloyed Steels: Process - Structure - Property Paradigm Venkata Vignesh Natarajan University of Texas at El Paso, [email protected] Follow this and additional works at: https://digitalcommons.utep.edu/open_etd Part of the Materials Science and Engineering Commons, and the Mechanics of Materials Commons Recommended Citation Natarajan, Venkata Vignesh, "Streamlining Alloy Design And Thermo-Mechanical Processing Parameters For High Strength Line Pipe Steels And Hot Rolled Microalloyed Steels: Process - Structure - Property Paradigm" (2018). Open Access Theses & Dissertations. 129. https://digitalcommons.utep.edu/open_etd/129 This is brought to you for free and open access by DigitalCommons@UTEP. It has been accepted for inclusion in Open Access Theses & Dissertations by an authorized administrator of DigitalCommons@UTEP. For more information, please contact [email protected]. STREAMLINING ALLOY DESIGN AND THERMO-MECHANICAL PROCESSING PARAMETERS FOR HIGH STRENGTH LINE PIPE STEELS AND HOT ROLLED MICROALLOYED STEELS: PROCESS – STRUCTURE – PROPERTY PARADIGM VENKATA VIGNESH NATARAJAN Doctoral Program in Materials Science and Engineering APPROVED: Devesh Misra, Ph.D., Chair Srinivasa Rao Singamaneni, Ph.D. Guikuan Yue, Ph.D. Charles Ambler, Ph.D. Dean of the Graduate School Copyright © by Venkata Vignesh Natarajan 2018 DEDICATION -

THEODORE WILLIAM RICHARDS January 31, 1868-April 2, 1928
NATIONAL ACADEMY OF SCIENCES T H E O D O R E W I L L I A M R ICHARDS 1868—1928 A Biographical Memoir by JAMES BRYANT CONANT Any opinions expressed in this memoir are those of the author(s) and do not necessarily reflect the views of the National Academy of Sciences. Biographical Memoir COPYRIGHT 1974 NATIONAL ACADEMY OF SCIENCES WASHINGTON D.C. THEODORE WILLIAM RICHARDS January 31, 1868-April 2, 1928 BY JAMES BRYANT CONANT HEODORE WILLIAM RICHARDS was a precocious son of distin- Tguished parents. He was born in Philadelphia on January 31, 1868, the third son and fifth child of William Trost Richards and Anna Matlack Richards, who had been married on June 30, 1856. As strict members of the Society of Friends, the Matlack family looked askance at a young man who earned his living painting pictures. Anna was "read out of meeting." The Quaker marriage ceremony took place in the house of a friend. The first months of the honeymoon were devoted to the com- position and illustration of a manuscript volume of poems for the lady who had first brought the young couple together. A mutual interest in Browning and Tennyson had started an acquaintanceship which rapidly became a romance. An old friend and fellow artist of Philadelphia reminiscing long after W. T. Richards had established his reputation as a landscape painter said, "He amazed me by getting married and resigning his position as designer [in a local firm manufacturing gas fixtures] in order to devote himself entirely to his art. -

From Physical Chemistry to Chemical Physics, 1913-1941
International Workshop on the History of Chemistry 2015 Tokyo From Physical Chemistry to Chemical Physics, 1913-1941 Jeremiah James Ludwig-Maximillian University, Munich, Germany There has never been one unique name for the intersection of chemistry and physics. Nor has it ever been defined by a single, stable set of methods. Nevertheless, it is possible and arguably rewarding to distinguish changes in the constellation of terms and techniques that have defined the intersection over the years. I will speak today about one such change, the advent and ascendancy of chemical physics in the interwar period. When the young Friedrich Wilhelm Ostwald first began to formulate his campaign for “physical chemistry” in 1877, he used the term almost interchangeably with two others, “general chemistry” and “theoretical chemistry.” According to his vision of what would soon become a new chemical discipline, physical chemistry would investigate and formulate the general principles that underlie all chemical reactions and phenomena. The primary strategy that he and his allies used to generate these principles was to formulate mathematical “laws” or “rules” generalizing the results of numerous experiments, often performed using measuring apparatus borrowed from physics. Their main fields of inquiry were thermochemistry and solution theory, and they avoided and often openly maligned speculations regarding structures or mechanisms that might underlie the macroscopic regularities embodied in their laws.1 In the first decades of the 20th-century, the modern atomic theory was firmly established, and with only a slight delay, the methods of 19th-century physical chemistry lost a considerable proportion of their audience. Theories relying upon atomistic thinking began to reshape the disciplinary intersections of chemistry and physics, and by the end of the 1930s, cutting-edge research into the general principles of chemistry looked quite different than it had at the turn of the century.