Notes Acids and Bases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
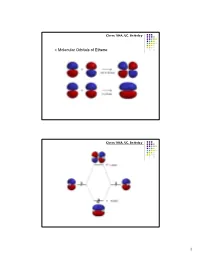
Π Molecular Orbitals of Ethene
Chem 104A, UC, Berkeley Molecular Orbitals of Ethene Chem 104A, UC, Berkeley 1 Chem 104A, UC, Berkeley Molecular Orbital Analysis of Ethene Dimerisation the reaction is said to be a "symmetry forbidden" – interestingly, this reaction is rare and very slow ! Chem 104A, UC, Berkeley Molecular Orbitals of 1,3-Butadiene 2 Chem 104A, UC, Berkeley Chem 104A, UC, Berkeley Molecular Orbital Analysis of Diels-Alder reaction 3 Chem 104A, UC, Berkeley Acid-Base Chemistry Reading : MT 6 Chem 104A, UC, Berkeley The Acid Base Theory of Brønsted and Lowry In 1923, within several months of each other, Johannes Nicolaus Brønsted (Denmark) and Thomas Martin Lowry (England) published essentially the same theory about how acids and bases behave. •An acid is a "proton donor." •A base is a "proton acceptor." 4 Chem 104A, UC, Berkeley + HCl + H2O <===> H3O + Cl¯ HCl - this is an acid, because it has a proton available to be transfered. H2O - this is a base, since it gets the proton that the acid lost. Now, here comes an interesting idea: + H3O - this is an acid, because it can give a proton. Cl¯ - this is a base, since it has the capacity to receive a proton. + Notice that each pair (HCl and Cl¯ as well as H2O and H3O differ by one proton (symbol = H+). These pairs are called conjugate pairs. Chem 104A, UC, Berkeley HCl + NaOH ---> H2O+ NaCl stronger stronger Weaker weaker conjugate conjugate acid base acid base + - NH4 +OH---> H2O+ NH3 stronger stronger weaker weaker conjugate conjugate acid base acid base 5 Chem 104A, UC, Berkeley H n A H 2O H n1 A H3O [H n1 A ][H3O ] pKa1 log [H n A] pKa 0 strong pKa 0 weak Chem 104A, UC, Berkeley Amphoteric Compound H n A H 2O H n1 A H3O NH 3 H 2O NH 4 OH 6 Chem 104A, UC, Berkeley Oxyacids AOp(OH)q A=Si, N, P, As, S, Se, Te, Cl, Br, I p=# of nonhydrogenated oxygen atom H + A ----O As atom A is rendered more positive, it becomes easier to break the O-H bond Because of enhanced bond polarization. -

Topic 5: Acids and Bases
Topic 5: Acids And BAses Topic 5: Acids and Bases C12-5-01 Outline the historical development of acid-base theories. Include: the Arrhenius, Brønsted-Lowry, and Lewis theories C12-5-02 Write balanced acid-base chemical equations. Include: conjugate acid-base pairs and amphoteric behaviour C12-5-03 Describe the relationship between the hydronium and hydroxide ion concentrations in water. Include: the ion product of water, Kw C12-5-04 Perform a laboratory activity to formulate an operational definition of pH . C12-5-05 Describe how an acid-base indicator works in terms of colour shifts and Le Ch âtelier’s principle. C12-5-06 Solve problems involving pH. C12-5-07 Distinguish between strong and weak acids and bases. Include: electrolytes and non-electrolytes C12-5-08 Write the equilibrium expression ( Ka or Kb) from a balanced chemical equation. C12-5-09 Use Ka or Kb to solve problems for pH, percent dissociation, and concentration. C12-5-10 Perform a laboratory activity to determine the concentration of an unknown acid or base, using a standardized acid or base. C12-5-11 Predict whether an aqueous solution of a given ionic compound will be acidic, basic, or neutral, given the formula. suggested Time: 14 hours Grade 12 C hemistry • Topic 5: Acids and Bases SpeCifiC Learning OutCOmeS Topic 5: C12-5-01: Outline the historical development of acid-base theories. Acids and include: the arrhenius, Br ønsted-Lowry, and Lewis theories Bases C12-5-02: Write balanced acid-base chemical equations. include: conjugate acid-base pairs and amphoteric behaviour (2 hours) 1 2 uggesTions for nsTrucTion 0 0 s i - - 5 5 - - Entry-Level Knowledge 2 2 1 1 In Grade 10 Science (S2-2-08), students experimented to classify acids and bases C C : : according to their characteristics. -

Tailoring the Surface Area and the Acid–Base Properties of Zro2 for Biodiesel Production from Nannochloropsis Sp
www.nature.com/scientificreports OPEN Tailoring the surface area and the acid–base properties of ZrO2 for biodiesel production from Nannochloropsis sp. Nurul Jannah Abd Rahman1, Anita Ramli1*, Khairulazhar Jumbri1,3 & Yoshimitsu Uemura2,3 Bifunctional heterogeneous catalysts have a great potential to overcome the shortcomings of homogeneous and enzymatic catalysts and simplify the biodiesel production processes using low-grade, high-free-fatty-acid feedstock. In this study, we developed ZrO2-based bifunctional heterogeneous catalysts for simultaneous esterifcation and transesterifcation of microalgae to biodiesel. To avoid the disadvantage of the low surface area of ZrO2, the catalysts were prepared via a surfactant-assisted sol-gel method, followed by hydrothermal treatments. The response surface methodology central composite design was employed to investigate various factors, like the surfactant/ Zr molar ratio, pH, aging time, and temperature on the ZrO2 surface area. The data were statistically analyzed to predict the optimal combination of factors, and further experiments were conducted for verifcation. Bi2O3 was supported on ZrO2 via the incipient wetness impregnation method. The catalysts were characterized by a variety of techniques, which disclosed that the surfactant-assisted ZrO2 nanoparticles possess higher surface area, better acid–base properties, and well-formed pore structures than bare ZrO2. The highest yield of fatty acid methyl esters (73.21%) was achieved using Bi2O3/ ZrO2(CTAB), and the catalytic activity of the developed catalysts was linearly correlated with the total densities of the acidic and basic sites. The mechanism of the simultaneous reactions was also discussed. Biodiesel is an attractive alternative source of energy owing to its renewability, biodegradability, sustainability, and non-toxicity1. -

Isomerization of Alpha-Pinene Oxide Over Solid Acid
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by ScholarBank@NUS ISOMERIZATION OF ALPHA-PINENE OXIDE OVER SOLID ACID CATALYSTS D. B. R. A. DE SILVA (B.Sc. (Hons.), UNIVERSITY OF PERADENIYA, SRI LANKA) A THESIS SUBMITTED FOR THE DEGREE OF MASTER OF SCIENCE DEPARTMENT OF CHEMISTRY NATIONAL UNIVERSITY OF SINGAPORE 2003 Acknowledgements First and foremost, I would like to thank my supervisor A/P G.K. Chuah, for her constant encouragement, invaluable guidance, patience and understanding throughout the length of my candidature in NUS. I am also grateful to A/P Stephan Jaenicke, for his invaluable guidance. I would also like to thank all the other members of our research group for their kind help and encouragement during my candidature. Thanks are due to my parents and wife for their understanding, encouragement and support. Finally, I wish to express my gratitude to the National University of Singapore for awarding me a valuable research scholarship. i TABLE OF CONTENTS PAGE Acknowledgement i Table of Contents ii Summary v List of Tables vii List of Figures ix Chapter I Introduction 1.1 Mesoporous Molecular Sieves 1 1.1.1 Catalytic Application of Mesoporous Materials 2 1.1.2 Modification of Mesoporous Materials 2 1.1.3 Micro- and Mesoporous Materials 4 1.2 Environment Impact of Solid Catalysts 6 1.3 Solid Acid and Catalysts 8 1.4 Supported Oxide Catalysts 9 1.4.1 Metal Oxide Supported Boron Oxide 13 1.4.2 SiO2-Supported ZrO2 Catalysts 15 1.4.3 InCl3-Supported Solid Acid Catalysts 16 1.5 Methodology -

Rational Design of Metal Oxide Solid Acids for Sugar Conversion
catalysts Review Rational Design of Metal Oxide Solid Acids for Sugar Conversion Atsushi Takagaki Department of Applied Chemistry, Faculty of Engineering, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan; [email protected]; Tel.: +81-92-802-6711 Received: 15 October 2019; Accepted: 24 October 2019; Published: 29 October 2019 Abstract: Aqueous-phase acid-catalyzed reactions are essential for the conversion of cellulose-based biomass into chemicals. Brønsted acid and Lewis acid play important roles for these reactions, including hydrolysis of saccharides, isomerization and epimerization of aldoses, conversion of d-glucose into 5-hydroxymethylfurfural, cyclodehydration of sugar alcohols and conversion of trioses into lactic acid. A variety of metal oxide solid acids has been developed and applied for the conversion of sugars so far. The catalytic activity is mainly dependent on the structures and types of solid acids. Amorphous metal oxides possess coordinatively unsaturated metal sites that function as Lewis acid sites while some crystal metal oxides have strong Brønsted acid sites. This review introduces several types of metal oxide solid acids, such as layered metal oxides, metal oxide nanosheet aggregates, mesoporous metal oxides, amorphous metal oxides and supported metal oxides for sugar conversions. Keywords: Sugars; cellulose; glucose; 5-hydroxymethylfurfural; lactic acid; solid acid; Brønsted acid; Lewis acid; metal oxide; water-tolerant catalyst 1. Introduction The fundamental features of solid acid catalysts are the reusability of the catalysts, their easy separation from the products and solvents and unnecessary neutralization, which are beneficial to saving energy and cost. Besides these, special requirements of the catalysts for sugar conversion are high recognition ability of sugar molecules and high tolerance against water. -

Acid-Base Catalysis Application of Solid Acid-Base Catalysts
Modern Methods in Heterogeneous Catalysis Research Acid-Base Catalysis Application of Solid Acid-Base Catalysts Annette Trunschke 18 February 2005 Outline 1. Introduction - basic principles 2. Substrates and products 3. Kinds of acid / base catalysts - examples 4. Characterization of surface acidity / basicity - examples 5. Acid catalyzed reactions 6. Base catalyzed reactions 7. Acid-base bifunctional catalysis 8. Summary and outlook Modern Methods in Heterogeneous Catalysis Research : 18/02/2005 2 1. Introduction - Basic definitions concept acid base - + Brønsted H2O OH + H - - Lewis FeCl3 + Cl [FeCl4] Hydrogen transfer reactions intermediates acid-catalyzed + H+ carbenium ions base-catalyzed + H+ carbanions Modern Methods in Heterogeneous Catalysis Research : 18/02/2005 3 1. Introduction - Basic definitions specific acid / base general acid / base catalysis catalysis + - active H3O or OH undissociated acid or base species groups; a variety of species may be simultaneously active reaction •in solution •gas phase reactions medium / •on the surface of •high reaction temperatures conditions a hydrated solid Advantages of solid acid-base catalysts: • Easier separation from the product • Possible reuse and regeneration • Fewer disposal problems • Non-corrosive and environmentally friendly (but not always!) Modern Methods in Heterogeneous Catalysis Research : 18/02/2005 4 2. Substrates and products solid acid catalysis solid base catalysis substrates •Alkanes, aromatics •Alkenes (components of crude •Alkynes petrolium) •Alkyl aromatics •Alkenes (products of •Carbonyl compounds petrolium cracking (FCC, steam cracking)) products •Gasoline components •Chemical intermediates •Chemical intermediates •Fine chemicals •Fine chemicals Industrial processes in 1999: 103 solid acids worldwide production by catalytic cracking: 500x106 tonnes/y 10 solid bases 14 solid acid-base bifunctional catalysts K.Tanabe, W.F.Hölderich, Applied Catalysis A 181 (1999) 399. -

Efficient Biodiesel Production Via Solid Superacid Catalysis
RSC Advances REVIEW Efficient biodiesel production via solid superacid catalysis: a critical review on recent breakthrough Cite this: RSC Adv.,2016,6,78351 Peter Adeniyi Alaba,*a Yahaya Muhammad Sanib and Wan Mohd Ashri Wan Daud*a Biodiesel produced from triglycerides and/or free fatty acids (FFAs) by transesterification and esterification has attracted immense attention during the past decades as a biodegradable, renewable and sustainable fuel. Currently, the use of solid superacid catalysts has proved a more efficient and “green” approach due to avoidance of environmental and corrosion problems and reduced product purification procedures. However, it is less viable economically because the reusability is low due to the lack of a hydrophilic/ hydrophobic balance in the reactions that involve the use of inedible feedstock with a high water content. Therefore, this study gives a critical review on recent strategies towards efficient and “green” production of biodiesel via solid superacid catalysis. The strategies discussed include alkyl-bridged Received 1st April 2016 organosilica moieties functionalized hybrid catalysis to improve the hydrothermal stability of superacid Accepted 2nd August 2016 catalysts, pre- and in situ water removal, and process intensification via temperature profile reduction. DOI: 10.1039/c6ra08399d The strategies enabled well-defined porosity and an excellent hydrophobicity/hydrophilicity balance, www.rsc.org/advances which suppressed deactivation by water and glycerol. 1. Introduction posed by fossil diesel. Some studies have revealed that biodiesel has become the most interesting alternative.1–3 The two major There is urgent need for alternative sources of energy due to the precursors of biodiesel are triglycerides and free fatty acids unrenewability, unsustainability and environmental threat (FFAs), which are obtained from edible oil, nonedible oil,4,5 animal fats and used vegetable oils.6 Previously, the most popular biodiesel produced was from edible oil. -

Acid Strength Measurements of Amberlyst 15 Resin, P-Xylene-2
Reaction Kinetics, Mechanisms and Catalysis https://doi.org/10.1007/s11144-019-01551-7 Acid strength measurements of Amberlyst 15 resin, p‑xylene‑2‑sulfonic acid and chlorosulfonic and sulfuric acid treated SiO2, Al2O3, TiO2 and MgO Marek Marczewski1 · Monika Aleksandrowicz1 · Ewelina Brzezińska1 · Maja Podlewska1 · Mateusz Rychlik1 · Sandra Żmuda1 · Urszula Ulkowska1 · Marek Gliński1 · Osazuwa Osawaru2 Received: 22 November 2018 / Accepted: 11 February 2019 © The Author(s) 2019 Abstract The measurements of the minimum acid strength (H Omin) required for the ini- tiation of cis-stilbene at 303 K and tert-butylbenzene at 313 K, 323 K and 333 K have enabled the establishment of a much more precise acid strength scale based on test reactions. HOmin values obtained were: − 9.0 (cis-stilbene, 303 K), − 9.3, − 9.5 and − 9.9 (tert-butylbenzene, 313 K, 323 K, 333 K). The updated method was used for the determination of the acid strength of diferent catalysts obtained in the reaction of chlorosulfonic and sulfuric acid on diferent oxide carriers. It was found that the acid strength values depended on the basicity of the studied supports. For the supports treated with chlorosulfonic acid the values of acid strength were: − 11.5 < HO ≤ − 10.8 in the case of the less basic SiO 2 and − 9.3 < HO ≤ − 9.0 for the more basic TiO2, Al2O3 and MgO. For the supports treated with sulfuric acid the acid strength was: -9.3 < HO ≤ -9.0 for the less basic SiO2 and TiO2, − 9.0 < HO ≤ − 7.9 for the more basic Al 2O3 and − 7.9 < HO ≤ − 6.9 for the most basic MgO. -

Brønsted and Lewis Solid Acid Catalysts in the Valorization of Citronellal
catalysts Article Brønsted and Lewis Solid Acid Catalysts in the Valorization of Citronellal Federica Zaccheria 1, Federica Santoro 1, Elvina Dhiaul Iftitah 2 and Nicoletta Ravasio 1,* 1 CNR Institute of Molecular Science and Technology, Via Golgi 19, 20133 Milano, Italy; [email protected] (F.Z.); [email protected] (F.S.) 2 Chemistry Department Faculty of Science, Brawijaya University Jl. Veteran, Malang 65145, Indonesia; [email protected] * Correspondence: [email protected]; Tel.: +39-02-5031-4382 Received: 30 July 2018; Accepted: 12 September 2018; Published: 22 September 2018 Abstract: Terpenes are valuable starting materials for the synthesis of molecules that are of interest to the flavor, fragrance, and pharmaceutical industries. However, most processes involve the use of mineral acids or homogeneous Lewis acid catalysts. Here, we report results obtained in the liquid-phase reaction of citronellal with anilines under heterogeneous catalysis conditions to give tricyclic compounds with interesting pharmacological activity. The terpenic aldehyde could be converted into octahydroacridines with a 92% yield through an intramolecular imino Diels–Alder reaction of the imine initially formed in the presence of an acidic clay such as Montmorillonite KSF. Selectivity to the desired product strongly depended on the acid sites distribution, with Brønsted acids favoring selectivity to octahydroacridine and formation of the cis isomer. Pure Lewis acids such as silica–alumina with a very low amount of alumina gave excellent results with electron-rich anilines like toluidine and p-anisidine. This protocol can be applied starting directly from essential oils such as kaffir lime oil, which has a high citronellal content. -

Optimization of Salix Carbonation Solid Acid Catalysts for One-Step Synthesis by Response Surface Method
applied sciences Article Optimization of Salix Carbonation Solid Acid Catalysts for One-Step Synthesis by Response Surface Method Ping Lu 1, Kebing Wang 1,* and Juhui Gong 2 1 College of Science, Inner Mongolia Agricultural University, Huhhot 010018, China; [email protected] 2 School of Chemistry and Chemical Engineering, Inner Mongolia University, Huhhot 010018, China; [email protected] * Correspondence: [email protected] Received: 16 March 2019; Accepted: 8 April 2019; Published: 12 April 2019 Featured Application: One of the major problems which we faced in this century was energy shortage, Salix carbon-based solid acid catalyst is a promising method for preparing biodiesel with low-cost catalysts to alleviate the energy crisis. Abstract: Salix carboniferous solid acid catalysts were successfully obtained via one-step carbonization and sulfonation of Salix psammophila in the presence of concentrated sulfuric acid, which was then used in the esterification reaction between oleic acid and methanol to prepare the biodiesel. The esterification rate of the catalyst obtained from the reaction indicated the catalytic performance of the catalyst. Afterwards, the recycling performance of the catalyst was optimized and characterized based on Fourier transform infrared spectrometer. The catalyst performance was examined and optimized through the response surface method, and the catalyst was determined and characterized based on scanning electron microscope (SEM), elemental analysis, thermogravimetric analysis, and infrared analysis. The results suggested that the optimal preparation conditions were as follows: reaction temperature of 125 ◦C, reaction time of 102 min, solid–liquid ratio of 17 g/100 mL, standing time of 30 min, and the highest conversion level of 94.15%. -

History and the Teaching of Chemistry. a Tribute to Thomas Lowry's Textbook
Educación Química (2016) 27, 175---181 educación Química www.educacionquimica.info REFLECTION History and the teaching of chemistry. A tribute to Thomas Lowry’s textbook ‘‘Historical Introduction to Chemistry’’ William B. Jensen Department of Chemistry, University of Cincinnati, Cincinnati, OH 45221-0172, United States Received 9 March 2016; accepted 30 March 2016 Available online 11 June 2016 KEYWORDS Abstract Through Thomas Lowry’s book written in 1915, Historical Introduction to Chemistry, Words history this paper shows how chemistry can be taught from its own history. of chemistry; All Rights Reserved © 2016 Universidad Nacional Autónoma de México, Facultad de Química. Chemistry teaching; This is an open access item distributed under the Creative Commons CC License BY-NC-ND 4.0. Historical approach PALABRAS CLAVE Historia y Ensenanza˜ de la Química. Un tributo al libro de Thomas Lowry Historia de la ‘‘Introducción Histórica a la Química’’ química; Resumen A través del libro de Thomas Lowry escrito en 1915, Historical Introduction to Chem- Ensenanza˜ de la química; istry, este artículo muestra la manera de cómo se puede ensenar˜ química a partir de su propia Aproximación historia. histórica Derechos Reservados © 2016 Universidad Nacional Autónoma de México, Facultad de Química. Este es un artículo de acceso abierto distribuido bajo los términos de la Licencia Creative Commons CC BY-NC-ND 4.0. Though much has been written advocating the use of his- introductory textbooks which claim to include some his- tory of chemistry in the teaching of introductory chemistry tory of chemistry, usually in the form of portraits of select courses at both the high school and college levels, there chemists from the past with short biographical captions have been surprisingly few attempts to write a proper text- attached or, in a few cases, supplementary boxes briefly book based on this approach. -

A Porous Brønsted Superacid As an Efficient and Durable Solid Catalyst
Journal of Materials Chemistry A PAPER View Article Online View Journal | View Issue A porous Brønsted superacid as an efficient and durable solid catalyst† Cite this: J. Mater. Chem. A,2018,6, 18712 Qi Sun,‡*a Kewei Hu,‡a Kunyue Leng,b Xianfeng Yi,c Briana Aguila, d Yinyong Sun, c Anmin Zheng, c Xiangju Meng,a Shengqian Mad and Feng-Shou Xiao ‡*a The development of catalysts able to assist industrial chemical transformations is a topic of high importance. In view of the versatile catalytic capabilities of acid catalysts, extensive research efforts are being made to develop porous superacid materials with a high density of accessible active sites to replace molecular acid catalysts. Herein, we report the rational development of a porous Brønsted superacid by combining important elements that target high strength acidity into one material, as demonstrated by grafting the sulfonic acid group onto a highly fluorinated porous framework, where the acid strength and stability are greatly enhanced by an electron-withdrawing environment provided by the polymer backbone, reminiscent of that seen in Nafion® resin. In addition, the densely arranged acid groups that are confined in the three-dimensional nanospace facilitate the transfer of hydrons, thereby further increasing the acidity. By virtue of the pore structure and strong acidity, this system exhibits Received 7th July 2018 excellent performance for a wide range of reactions, far outperforming commercial acid resins under Accepted 12th September 2018 repeated batch and flow reaction conditions. Our findings demonstrate how this synthetic approach may DOI: 10.1039/c8ta06516k instruct the future design of heterogeneous acid catalysts with advantageous reaction capabilities and rsc.li/materials-a stability.