Lewis and Bronsted Concept of Acids and Bases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
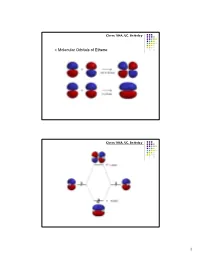
Π Molecular Orbitals of Ethene
Chem 104A, UC, Berkeley Molecular Orbitals of Ethene Chem 104A, UC, Berkeley 1 Chem 104A, UC, Berkeley Molecular Orbital Analysis of Ethene Dimerisation the reaction is said to be a "symmetry forbidden" – interestingly, this reaction is rare and very slow ! Chem 104A, UC, Berkeley Molecular Orbitals of 1,3-Butadiene 2 Chem 104A, UC, Berkeley Chem 104A, UC, Berkeley Molecular Orbital Analysis of Diels-Alder reaction 3 Chem 104A, UC, Berkeley Acid-Base Chemistry Reading : MT 6 Chem 104A, UC, Berkeley The Acid Base Theory of Brønsted and Lowry In 1923, within several months of each other, Johannes Nicolaus Brønsted (Denmark) and Thomas Martin Lowry (England) published essentially the same theory about how acids and bases behave. •An acid is a "proton donor." •A base is a "proton acceptor." 4 Chem 104A, UC, Berkeley + HCl + H2O <===> H3O + Cl¯ HCl - this is an acid, because it has a proton available to be transfered. H2O - this is a base, since it gets the proton that the acid lost. Now, here comes an interesting idea: + H3O - this is an acid, because it can give a proton. Cl¯ - this is a base, since it has the capacity to receive a proton. + Notice that each pair (HCl and Cl¯ as well as H2O and H3O differ by one proton (symbol = H+). These pairs are called conjugate pairs. Chem 104A, UC, Berkeley HCl + NaOH ---> H2O+ NaCl stronger stronger Weaker weaker conjugate conjugate acid base acid base + - NH4 +OH---> H2O+ NH3 stronger stronger weaker weaker conjugate conjugate acid base acid base 5 Chem 104A, UC, Berkeley H n A H 2O H n1 A H3O [H n1 A ][H3O ] pKa1 log [H n A] pKa 0 strong pKa 0 weak Chem 104A, UC, Berkeley Amphoteric Compound H n A H 2O H n1 A H3O NH 3 H 2O NH 4 OH 6 Chem 104A, UC, Berkeley Oxyacids AOp(OH)q A=Si, N, P, As, S, Se, Te, Cl, Br, I p=# of nonhydrogenated oxygen atom H + A ----O As atom A is rendered more positive, it becomes easier to break the O-H bond Because of enhanced bond polarization. -

Structure and Bonding Electron Configurations in the Periodic Table
Structure and Bonding The study of organic chemistry must at some point extend to the molecular level, for the physical and chemical properties of a substance are ultimately explained in terms of the structure and bonding of molecules. This module introduces some basic facts and principles that are needed for a discussion of organic molecules. Electronic Configurations Electron Configurations in the Periodic Table 1A 2A 3A 4A 5A 6A 7A 8A 1 2 H He 1 2 1s 1s 3 4 5 6 7 8 9 10 Li Be B C N O F Ne 2 2 2 2 2 2 2 2 1s 1s 1s 1s 1s 1s 1s 1s 2s1 2s2 2s22p1 2s22p2 2s22p3 2s22p4 2s22p5 2s22p6 11 12 13 14 15 16 17 18 Na Mg Al Si P S Cl Ar [Ne] [Ne] [Ne] [Ne] [Ne] [Ne] [Ne] [Ne] 3s1 3s2 3s23p1 3s23p2 3s23p3 3s23p4 3s23p5 3s23p6 Four elements, hydrogen, carbon, oxygen and nitrogen, are the major components of most organic compounds. Consequently, our understanding of organic chemistry must have, as a foundation, an appreciation of the electronic structure and properties of these elements. The truncated periodic table shown above provides the orbital electronic structure for the first eighteen elements (hydrogen through argon). According to the Aufbau principle, the electrons of an atom occupy quantum levels or orbitals starting from the lowest energy level, and proceeding to the highest, with each orbital holding a maximum of two paired electrons (opposite spins). Electron shell #1 has the lowest energy and its s-orbital is the first to be filled. -

VSEPR Theory
VSEPR Theory The valence-shell electron-pair repulsion (VSEPR) model is often used in chemistry to predict the three dimensional arrangement, or the geometry, of molecules. This model predicts the shape of a molecule by taking into account the repulsion between electron pairs. This handout will discuss how to use the VSEPR model to predict electron and molecular geometry. Here are some definitions for terms that will be used throughout this handout: Electron Domain – The region in which electrons are most likely to be found (bonding and nonbonding). A lone pair, single, double, or triple bond represents one region of an electron domain. H2O has four domains: 2 single bonds and 2 nonbonding lone pairs. Electron Domain may also be referred to as the steric number. Nonbonding Pairs Bonding Pairs Electron domain geometry - The arrangement of electron domains surrounding the central atom of a molecule or ion. Molecular geometry - The arrangement of the atoms in a molecule (The nonbonding domains are not included in the description). Bond angles (BA) - The angle between two adjacent bonds in the same atom. The bond angles are affected by all electron domains, but they only describe the angle between bonding electrons. Lewis structure - A 2-dimensional drawing that shows the bonding of a molecule’s atoms as well as lone pairs of electrons that may exist in the molecule. Provided by VSEPR Theory The Academic Center for Excellence 1 April 2019 Octet Rule – Atoms will gain, lose, or share electrons to have a full outer shell consisting of 8 electrons. When drawing Lewis structures or molecules, each atom should have an octet. -

Practical Course in General and Inorganic Chemistry III
Practical course in general and inorganic chemistry III. Common acid-base theories The Arrhenius definition - Dissociation of strong/weak acids and bases - Preparation of salts - The limitations of the Arrhenius acid-base theory The Brønsted-Lowry definition - Relative strengths of Brønsted-Lowry acids and bases - Amphiprotic species (the amphoterism) - Types of acid-base reactions - Structures of different boric acids and borax - Preparation of normal salts - Acid salts The Arrhenius acid-base theory (1884) • Acid : dissociate in aqueous solution form hydrogen + or the later-termed oxonium (H 3O ) ions univalent, strong acid: univalent, weak acid: diprotic, strong acid: diprotic, weak acid: triprotic, weak acid: • Stepwise acid dissociation : 1 Relative strength of inorganic oxoacids (Pauling) − + + Om X(OH) n Om+1X(OH) n−1 H acid anhydride m = 3 very strong acid m = 2 strong acid m = 1 weak acid m = 0 very weak acid Arrhenius-bases and anhydrides • Base : in aqueous solution form hydroxide (OH −) ion(s) and cation(s) univalent, strong base: divalent, strong base: univalent, weak base: divalent, weak base: trivalent, weak base: • Base anhydrides = metal-oxides : derived from the base by subtracting the molecules of water • base anhydrides of strong bases: 2 Preparation of salts (Arrhenius) base + acid = salt + water • Preparation of salt from a strong base: • Preparation of salt from a weak base : • If the acid is partially neutralized: acid salt The limitations of the Arrhenius acid-base theory • Although ammonia is well-known as a -

Acids Lewis Acids and Bases Lewis Acids Lewis Acids: H+ Cu2+ Al3+ Lewis Bases
E5 Lewis Acids and Bases (Session 1) Acids November 5 - 11 Session one Bronsted: Acids are proton donors. • Pre-lab (p.151) due • Problem • 1st hour discussion of E4 • Compounds containing cations other than • Lab (Parts 1and 2A) H+ are acids! Session two DEMO • Lab: Parts 2B, 3 and 4 Problem: Some acids do not contain protons Lewis Acids and Bases Example: Al3+ (aq) = ≈ pH 3! Defines acid/base without using the word proton: + H H H • Cl-H + • O Cl- • • •• O H H Acid Base Base Acid . A BASE DONATES unbonded ELECTRON PAIR/S. An ACID ACCEPTS ELECTRON PAIR/S . Deodorants and acid loving plant foods contain aluminum salts Lewis Acids Lewis Bases . Electron rich species; electron pair donors. Electron deficient species ; potential electron pair acceptors. Lewis acids: H+ Cu2+ Al3+ “I’m deficient!” Ammonia hydroxide ion water__ (ammine) (hydroxo) (aquo) Acid 1 Lewis Acid-Base Reactions Lewis Acid-Base Reactions Example Metal ion + BONDED H H H + • to water H + • O O • • •• molecules H H Metal ion Acid + Base Complex ion surrounded by water . The acid reacts with the base by bonding to one molecules or more available electron pairs on the base. The acid-base bond is coordinate covalent. The product is a complex or complex ion Lewis Acid-Base Reaction Products Lewis Acid-Base Reaction Products Net Reaction Examples Net Reaction Examples 2+ 2+ + + Pb + 4 H2O [Pb(H2O)4] H + H2O [H(H2O)] Lewis acid Lewis base Tetra aquo lead ion Lewis acid Lewis base Hydronium ion 2+ 2+ 2+ 2+ Ni + 6 H2O [Ni(H2O)6] Cu + 4 H2O [Cu(H2O)4] Lewis acid Lewis base Hexa aquo nickel ion Lewis acid Lewis base Tetra aquo copper(II)ion DEMO DEMO Metal Aquo Complex Ions Part 1. -

In This Section and in the Next Section on Redox Chemistry, We Will Often Draw on Fundamental Thermodynamic Concepts
Prerequisites for Section III (1-2 weeks) • In this section and in the next section on redox chemistry, we will often draw on fundamental thermodynamic concepts. You should recall the meaning of ∆H, ∆S, and ∆G for chemical and physical changes — and try to think about systems using these concepts. • ∆G˚ = –RTln Keq This relationship is central to relating chemical thermodynamics and equilibrium. • ∆G = ∆H – T∆S for a process with a specified T and P • The above two relationships can be combined to provide invaluable guidance in understanding how a chemist can manipulate ∆H or ∆S (say, by making changes in ∆S˚/R –∆H˚/RT solvents or molecular substituents) to dramatically shift equilibria: Keq = e e . At room temperature, RT ≈ 2.5 kJ/mol, so this equation means that, for example, a change in solvent that causes ∆H to shift by only ~ 6 kJ/mol will shift the equilibrium constant for a reaction by a factor of 10 (roughly). Acids and Bases a. Brønsted Acidity — aqueous equilibria, solvent leveling, periodic trends, oxoacids, anhydrous acids and bases, amphoterism, polyoxo ions, nonaqueous solvents • Brønsted acid = proton donor, Brønsted base = proton acceptor + • pH = –log[H ], pKa = –logKa; pKb = –logKb • [H+][H+] = 10–14 in aqueous solutions • pKa + pKb = 14 – for any conjugate acid-base pair in dilute aqueous solution • recall concepts of conjugate acids and bases; strong acids have weak conjugate bases, weak acids have strong conjugate bases • Things that affect acidity and basicity of organic acids and bases: the ability of a conjugate base (acid) to delocalize negative (positive) charge through inductive effects or resonance affects the strength of an acid (base). -

8.3 Bonding Theories >
8.3 Bonding Theories > Chapter 8 Covalent Bonding 8.1 Molecular Compounds 8.2 The Nature of Covalent Bonding 8.3 Bonding Theories 8.4 Polar Bonds and Molecules 1 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals Molecular Orbitals How are atomic and molecular orbitals related? 2 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals • The model you have been using for covalent bonding assumes the orbitals are those of the individual atoms. • There is a quantum mechanical model of bonding, however, that describes the electrons in molecules using orbitals that exist only for groupings of atoms. 3 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals • When two atoms combine, this model assumes that their atomic orbitals overlap to produce molecular orbitals, or orbitals that apply to the entire molecule. 4 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals Just as an atomic orbital belongs to a particular atom, a molecular orbital belongs to a molecule as a whole. • A molecular orbital that can be occupied by two electrons of a covalent bond is called a bonding orbital. 5 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals Sigma Bonds When two atomic orbitals combine to form a molecular orbital that is symmetrical around the axis connecting two atomic nuclei, a sigma bond is formed. • Its symbol is the Greek letter sigma (σ). -

Lesson 21: Acids & Bases
Lesson 21: Acids & Bases - Far From Basic Lesson Objectives: • Students will identify acids and bases by the Lewis, Bronsted-Lowry, and Arrhenius models. • Students will calculate the pH of given solutions. Getting Curious You’ve probably heard of pH before. Many personal hygiene products make claims about pH that are sometimes based on true science, but frequently are not. pH is the measurement of [H+] ion concentration in any solution. Generally, it can tell us about the acidity or alkaline (basicness) of a solution. Click on the CK-12 PLIX Interactive below for an introduction to acids, bases, and pH, and then answer the questions below. Directions: Log into CK-12 as follows: Username - your Whitmore School Username Password - whitmore2018 Questions: Copy and paste questions 1-3 in the Submit Box at the bottom of this page, and answer the questions before going any further in the lesson: 1. After dragging the small white circles (in this simulation, the indicator papers) onto each substance, what do you observe about the pH of each substance? 2. If you were to taste each substance (highly inadvisable in the chemistry laboratory!), how would you imagine they would taste? 3. Of the three substances, which would you characterize as acid? Which as basic? Which as neutral? Use the observed pH levels to support your hypotheses. Chemistry Time Properties of Acids and Bases Acids and bases are versatile and useful materials in many chemical reactions. Some properties that are common to aqueous solutions of acids and bases are listed in the table below. Acids Bases conduct electricity in solution conduct electricity in solution turn blue litmus paper red turn red litmus paper blue have a sour taste have a slippery feeling react with acids to create a neutral react with bases to create a neutral solution solution react with active metals to produce hydrogen gas Note: Litmus paper is a type of treated paper that changes color based on the acidity of the solution it comes in contact with. -

Chemical Bonding & Chemical Structure
Chemistry 201 – 2009 Chapter 1, Page 1 Chapter 1 – Chemical Bonding & Chemical Structure ings from inside your textbook because I normally ex- Getting Started pect you to read the entire chapter. 4. Finally, there will often be a Supplement that con- If you’ve downloaded this guide, it means you’re getting tains comments on material that I have found espe- serious about studying. So do you already have an idea cially tricky. Material that I expect you to memorize about how you’re going to study? will also be placed here. Maybe you thought you would read all of chapter 1 and then try the homework? That sounds good. Or maybe you Checklist thought you’d read a little bit, then do some problems from the book, and just keep switching back and forth? That When you have finished studying Chapter 1, you should be sounds really good. Or … maybe you thought you would able to:1 go through the chapter and make a list of all of the impor- tant technical terms in bold? That might be good too. 1. State the number of valence electrons on the following atoms: H, Li, Na, K, Mg, B, Al, C, Si, N, P, O, S, F, So what point am I trying to make here? Simply this – you Cl, Br, I should do whatever you think will work. Try something. Do something. Anything you do will help. 2. Draw and interpret Lewis structures Are some things better to do than others? Of course! But a. Use bond lengths to predict bond orders, and vice figuring out which study methods work well and which versa ones don’t will take time. -

Electron Configurations, Orbital Notation and Quantum Numbers
5 Electron Configurations, Orbital Notation and Quantum Numbers Electron Configurations, Orbital Notation and Quantum Numbers Understanding Electron Arrangement and Oxidation States Chemical properties depend on the number and arrangement of electrons in an atom. Usually, only the valence or outermost electrons are involved in chemical reactions. The electron cloud is compartmentalized. We model this compartmentalization through the use of electron configurations and orbital notations. The compartmentalization is as follows, energy levels have sublevels which have orbitals within them. We can use an apartment building as an analogy. The atom is the building, the floors of the apartment building are the energy levels, the apartments on a given floor are the orbitals and electrons reside inside the orbitals. There are two governing rules to consider when assigning electron configurations and orbital notations. Along with these rules, you must remember electrons are lazy and they hate each other, they will fill the lowest energy states first AND electrons repel each other since like charges repel. Rule 1: The Pauli Exclusion Principle In 1925, Wolfgang Pauli stated: No two electrons in an atom can have the same set of four quantum numbers. This means no atomic orbital can contain more than TWO electrons and the electrons must be of opposite spin if they are to form a pair within an orbital. Rule 2: Hunds Rule The most stable arrangement of electrons is one with the maximum number of unpaired electrons. It minimizes electron-electron repulsions and stabilizes the atom. Here is an analogy. In large families with several children, it is a luxury for each child to have their own room. -

Inorganic Chemistry for Dummies® Published by John Wiley & Sons, Inc
Inorganic Chemistry Inorganic Chemistry by Michael L. Matson and Alvin W. Orbaek Inorganic Chemistry For Dummies® Published by John Wiley & Sons, Inc. 111 River St. Hoboken, NJ 07030-5774 www.wiley.com Copyright © 2013 by John Wiley & Sons, Inc., Hoboken, New Jersey Published by John Wiley & Sons, Inc., Hoboken, New Jersey Published simultaneously in Canada No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning or otherwise, except as permitted under Sections 107 or 108 of the 1976 United States Copyright Act, without either the prior written permis- sion of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923, (978) 750-8400, fax (978) 646-8600. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, (201) 748-6011, fax (201) 748-6008, or online at http://www.wiley. com/go/permissions. Trademarks: Wiley, the Wiley logo, For Dummies, the Dummies Man logo, A Reference for the Rest of Us!, The Dummies Way, Dummies Daily, The Fun and Easy Way, Dummies.com, Making Everything Easier, and related trade dress are trademarks or registered trademarks of John Wiley & Sons, Inc. and/or its affiliates in the United States and other countries, and may not be used without written permission. All other trade- marks are the property of their respective owners. John Wiley & Sons, Inc., is not associated with any product or vendor mentioned in this book. -

Atomic Structure and Bonding
IM2665 Chemistry of Nanomaterials Atomic Structure and Bonding Assoc. Prof. Muhammet Toprak Division of Functional Materials KTH Royal Institute of Technology Background • Electromagnetic waves • Materials wave motion, • Quantified energy and – Louis de Broglie (1892-1987) photons • Uncertainity principle – Max Planck (1858-1947) – Werner Heisenberg (1901-1976) – Albert Einstein (1879-1955) • Schrödinger equation • Bohr’s atom model – Erwin Schrödinger (1887-1961) – Niels Bohr (1885-1962) IM2657 Nanostr. Mater. & Self Assembly 2 Electromagnetic Spectrum Visible light is only a small part of the Electromagnetic Spectrum IM2657 Nanostr. Mater. & Self Assembly 3 Electromagnetic Waves • Wavemotion is defined by • Calculations – ν = Frequency ( Hz) – c = ν × λ – λ = wavelength (m) – c = 3,00 × 108 m/s (speed of light) IM2657 Nanostr. Mater. & Self Assembly 4 Quantified Energy and Photons • E = h × ν; where h = 6,63 × 10-34 J s (Planck’s constant) • Photoelectric Effect (1905) IM2657 Nanostr. Mater. & Self Assembly 5 Thomson´s Pudding Model For a helium atom, the model proposes a large spherical cloud with two units of positive charge. Th e two electrons lie on a line through the center of the cloud. The loss of one electron produces the He+1 ion, with the remaining electron at the center of the cloud. The loss of a second electron prod uces He+2 , in which there is just a cloud of positive charge. IM2657 Nanostr. Mater. & Self Assembly 6 Rutherford’s Experiment The notion that atoms consist of very small nuclei containing protons and neutrons surrounded by a much larger cloud of electrons was IM2657 Nanostr. Mater. & Self Assembly 7 developed from an α particle scattering experiment.