Thomas Martin Lowry and the Mixed Multiple Bond
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
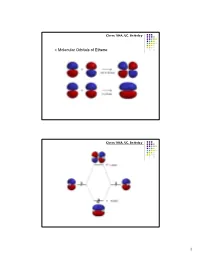
Π Molecular Orbitals of Ethene
Chem 104A, UC, Berkeley Molecular Orbitals of Ethene Chem 104A, UC, Berkeley 1 Chem 104A, UC, Berkeley Molecular Orbital Analysis of Ethene Dimerisation the reaction is said to be a "symmetry forbidden" – interestingly, this reaction is rare and very slow ! Chem 104A, UC, Berkeley Molecular Orbitals of 1,3-Butadiene 2 Chem 104A, UC, Berkeley Chem 104A, UC, Berkeley Molecular Orbital Analysis of Diels-Alder reaction 3 Chem 104A, UC, Berkeley Acid-Base Chemistry Reading : MT 6 Chem 104A, UC, Berkeley The Acid Base Theory of Brønsted and Lowry In 1923, within several months of each other, Johannes Nicolaus Brønsted (Denmark) and Thomas Martin Lowry (England) published essentially the same theory about how acids and bases behave. •An acid is a "proton donor." •A base is a "proton acceptor." 4 Chem 104A, UC, Berkeley + HCl + H2O <===> H3O + Cl¯ HCl - this is an acid, because it has a proton available to be transfered. H2O - this is a base, since it gets the proton that the acid lost. Now, here comes an interesting idea: + H3O - this is an acid, because it can give a proton. Cl¯ - this is a base, since it has the capacity to receive a proton. + Notice that each pair (HCl and Cl¯ as well as H2O and H3O differ by one proton (symbol = H+). These pairs are called conjugate pairs. Chem 104A, UC, Berkeley HCl + NaOH ---> H2O+ NaCl stronger stronger Weaker weaker conjugate conjugate acid base acid base + - NH4 +OH---> H2O+ NH3 stronger stronger weaker weaker conjugate conjugate acid base acid base 5 Chem 104A, UC, Berkeley H n A H 2O H n1 A H3O [H n1 A ][H3O ] pKa1 log [H n A] pKa 0 strong pKa 0 weak Chem 104A, UC, Berkeley Amphoteric Compound H n A H 2O H n1 A H3O NH 3 H 2O NH 4 OH 6 Chem 104A, UC, Berkeley Oxyacids AOp(OH)q A=Si, N, P, As, S, Se, Te, Cl, Br, I p=# of nonhydrogenated oxygen atom H + A ----O As atom A is rendered more positive, it becomes easier to break the O-H bond Because of enhanced bond polarization. -

Notes Acids and Bases
Notes Acids and Bases Arrhenius Acid Base Theory: In 1884 Svante Arrhenius proposed the first theoretical model for acids and bases. Prior to that time, these chemically opposite substances were described in properties such as their taste; their effects on metals, carbonates, and dyes (called indicators); their feel to the touch, and their ability to react with each other. According to the Arrhenius theory , pure water dissociates to some extent to produce hydrogen ions, H + and hydroxide ions, OH -. When this occurs, equal amounts of H + and OH - ions are produced: + - H2O(l) H (aq) + OH (aq) An acid, according to Arrhenius, is any substance that liberates H + ions when placed in water. When the H + concentration is elevated the OH - concentration decreases, this solution is said to be acidic. Similarly, a base is defined as any substance that liberates OH - ions when placed in water. The resulting solution has a higher concentration of OH - ions than H + ions and is said to be basic, or alkaline. + - ACID: HCl (aq) H (aq) + Cl (aq) + - BASE: NaOH (aq) Na (aq) + OH (aq) Brønsted - Lowry Acid Base Theory: A more general and realistic theory was proposed by two chemists working independent of the other, Johannes Nicolaus Brønsted of Denmark and Thomas Martin Lowry of England. Both chemists are given credit; the theory is named the Brønsted-Lowry theory . Acids are defined as substances that donate H + ions, protons and therefore are called proton donators, while bases are substances that accept protons and are defined as proton acceptors. On this basis, the autoionization of water is given as follows: + - H2O(l) + H 2O(l) H3O (aq) + OH (aq) In this reaction, one water molecule donates a proton, H +, and behaves as an acid, while the other water molecule accepts the proton and behaves as a base. -

Recipients of Honoris Causa Degrees and of Scholarships and Awards Honoris Causa Degrees of the University of Melbourne*
Recipients of Honoris Causa Degrees and of Scholarships and Awards Honoris Causa Degrees of the University of Melbourne* MEMBERS OF THE ROYAL FAMILY 1868 His Royal Highness Prince Alfred Ernest Albert, Duke of Edinburgh (Edinburgh) LL.D. 1901 His Royal Highness Prince George Frederick Ernest Albert, Duke of York (afterwards King George V) (Cambridge) LL.D. 1920 His Royal Highness Edward Albert Christian George Andrew Patrick David, Prince of Wales (afterwards King Edward VIII) (Oxford) LL.D. 1927 His Royal Highness Prince Albert Frederick Arthur George, Duke of York (afterwards King George VI) (Cambridge) LL.D. 1934 His Royal Highness Prince Henry William Frederick Albert, Duke of Gloucester (Cambridge) LL.D. 1958 Her Majesty Queen Elizabeth, The Queen Mother (Oxford) LL.D. OTHER DISTINGUISHED GRADUATES 1914 Charles Greely Abbot (Massachusetts) D.Sc. Henry Edward Armstrong (Leipzig) D.Sc. William Bateson (Cambridge) D.Sc. William Morris Davis (Harvard) D.Sc. Frank Watson Dyson (Cambridge) D.Sc. Sir Thomas Henry Holland (Calcutta) D.Sc. Luigi Antonio Ettore Luiggi (Genoa) D.Sc. William Jackson Pope (Cambridge) D.Sc. Alfred William Porter (London) D.Sc. Sir Ernest Rutherford (Cambridge and New Zealand) D.Sc. Sir Edward Albert Schafer (London) D.Sc. Johannes Walther (Jena) D.Sc. 1915 Robert Randolph Garran (Sydney) M.A. Albert Bathurst Piddington (Sydney) M.A. 1918 Andre Siegfried (Paris) Litt.D. 1920 Sir William Riddell Birdwood (Cambridge) LL.D. Sir John Monash (Oxford and Cambridge) LL.D. 1927 Stanley Melbourne Bruce (Cambridge) LL.D. 1931 Charles Edwin Woodrow Bean (Oxford) Litt.D. 1932 Charles Herbert Fagge (London) M.D. 1934 Sir John Cadman (Birmingham) D.Eng. -

History and the Teaching of Chemistry. a Tribute to Thomas Lowry's Textbook
Educación Química (2016) 27, 175---181 educación Química www.educacionquimica.info REFLECTION History and the teaching of chemistry. A tribute to Thomas Lowry’s textbook ‘‘Historical Introduction to Chemistry’’ William B. Jensen Department of Chemistry, University of Cincinnati, Cincinnati, OH 45221-0172, United States Received 9 March 2016; accepted 30 March 2016 Available online 11 June 2016 KEYWORDS Abstract Through Thomas Lowry’s book written in 1915, Historical Introduction to Chemistry, Words history this paper shows how chemistry can be taught from its own history. of chemistry; All Rights Reserved © 2016 Universidad Nacional Autónoma de México, Facultad de Química. Chemistry teaching; This is an open access item distributed under the Creative Commons CC License BY-NC-ND 4.0. Historical approach PALABRAS CLAVE Historia y Ensenanza˜ de la Química. Un tributo al libro de Thomas Lowry Historia de la ‘‘Introducción Histórica a la Química’’ química; Resumen A través del libro de Thomas Lowry escrito en 1915, Historical Introduction to Chem- Ensenanza˜ de la química; istry, este artículo muestra la manera de cómo se puede ensenar˜ química a partir de su propia Aproximación historia. histórica Derechos Reservados © 2016 Universidad Nacional Autónoma de México, Facultad de Química. Este es un artículo de acceso abierto distribuido bajo los términos de la Licencia Creative Commons CC BY-NC-ND 4.0. Though much has been written advocating the use of his- introductory textbooks which claim to include some his- tory of chemistry in the teaching of introductory chemistry tory of chemistry, usually in the form of portraits of select courses at both the high school and college levels, there chemists from the past with short biographical captions have been surprisingly few attempts to write a proper text- attached or, in a few cases, supplementary boxes briefly book based on this approach. -

The Royal Society of Chemistry Presidents 1841 T0 2021
The Presidents of the Chemical Society & Royal Society of Chemistry (1841–2024) Contents Introduction 04 Chemical Society Presidents (1841–1980) 07 Royal Society of Chemistry Presidents (1980–2024) 34 Researching Past Presidents 45 Presidents by Date 47 Cover images (left to right): Professor Thomas Graham; Sir Ewart Ray Herbert Jones; Professor Lesley Yellowlees; The President’s Badge of Office Introduction On Tuesday 23 February 1841, a meeting was convened by Robert Warington that resolved to form a society of members interested in the advancement of chemistry. On 30 March, the 77 men who’d already leant their support met at what would be the Chemical Society’s first official meeting; at that meeting, Thomas Graham was unanimously elected to be the Society’s first president. The other main decision made at the 30 March meeting was on the system by which the Chemical Society would be organised: “That the ordinary members shall elect out of their own body, by ballot, a President, four Vice-Presidents, a Treasurer, two Secretaries, and a Council of twelve, four of Introduction whom may be non-resident, by whom the business of the Society shall be conducted.” At the first Annual General Meeting the following year, in March 1842, the Bye Laws were formally enshrined, and the ‘Duty of the President’ was stated: “To preside at all Meetings of the Society and Council. To take the Chair at all ordinary Meetings of the Society, at eight o’clock precisely, and to regulate the order of the proceedings. A Member shall not be eligible as President of the Society for more than two years in succession, but shall be re-eligible after the lapse of one year.” Little has changed in the way presidents are elected; they still have to be a member of the Society and are elected by other members. -

MAUVE and ITS ANNIVERSARIES* Anthony S
Bull. Hist. Chem., VOLUME 32, Number 1 (2007) 35 MAUVE AND ITS ANNIVERSARIES* Anthony S. Travis, Edelstein Center, Hebrew University of Jerusalem/Leo Baeck Institute London Introduction chemical constitution of the natural dye indigo, as well as other natural products. Of particular interest, however, In 1856, William Henry Perkin in were the components of, and pos- London prepared the first aniline sible uses for, the vast amount of dye, later known as mauve. The coal-tar waste available from coal- eighteen-year-old inventor sought, gas works and distilleries. Around but failed, to find a licensee for 1837, Liebig’s assistant A. Wilhelm his process, and then embarked Hofmann extracted several nitro- on manufacture, with the back- gen-containing oils from coal tar ing of his father and a brother. and showed that of these bases the The opening of their factory and one present in greatest abundance the sudden demand for mauve in was identical with a product earlier 1859 foreshadowed the growth obtained from indigo as well as of the modern organic chemical from other sources. It was soon industry. The search throughout known as aniline. Europe for novel colorants made In 1845 Hofmann moved to scientific reputations and trans- London to head the new Royal Col- formed the way in which research lege of Chemistry (RCC). There he was conducted, in both academic continued his studies into aniline and industrial laboratories. Ac- and its reactions. At that time, there cordingly, the sesquicentennial William Henry Perkin (1838-1907), in 1860. Heinrich Caro (1834-1910), technical leader were no modern structural formulae of mauve provides an opportune at BASF, 1868-1889. -

J. R. Partington (1886-1965): Physical Chemistry in Deed and Word
Bull. Hist. Chem., VOLUME 34, Number 1 (2009) 11 J. R. PARTINGTON (1886-1965): PHYSICAL CHEMISTRY IN DEED AND WORD William H. Brock, University of Leicester, UK Introduction Scottish tailor. While he was still quite young his parents moved to the seaside town of Southport, to the north of Today James Riddick Partington (1886-1965) is remem- Liverpool, allowing Partington the benefit of education bered as an historian of chemistry rather than as the at the Victoria Science and Art School that had opened significant British research chemist and textbook writer in 1887 (1). Here his prowess as a mathematician and he was perceived to be in the 1920s and 1930s. Because practical chemist must have been forged. He left school his textbooks were specifically geared to the British in 1901 when he was 15 because his parents moved back secondary school and university systems, he is prob- to Bolton. There he began to assist the town’s Public ably not well known in the United States as a textbook Analyst, a post that must have involved the acquirement writer. Nor, in America or in Europe, is he remembered of the skills in volumetric and gravimetric analysis that as a practicing physical chemist who made contributions were a hallmark of his later work. After a couple of years, to thermodynamics, the determination of specific heats, and still in local government employment, he became a and to electrochemical theory. So, for example, he is not laboratory assistant in the town’s Pupil Teachers Train- mentioned in Keith Laidler’s World of Physical Chemis- ing College before finally becoming a clerk in Bolton’s try (1993). -

Lewis and Bronsted Concept of Acids and Bases
Lewis and Bronsted Concept of Acids and Bases Lewis Concept : Acids and bases are an important part of chemistry. One of the most applicable theories is the Lewis acid/base motif that extends the definition of an acid and base beyond H+ and OH- ions as described by Brønsted-Lowry acids and bases. Introduction The Brønsted acid-base theory has been used throughout the history of acid and base chemistry. However, this theory is very restrictive and focuses primarily on acids and bases acting as proton donors and acceptors. Sometimes conditions arise where the theory doesn't necessarily fit, such as in solids and gases. In 1923, G.N. Lewis from UC Berkeley proposed an alternate theory to describe acids and bases. His theory gave a generalized explanation of acids and bases based on structure and bonding. Through the use of the Lewis definition of acids and bases, chemists are now able to predict a wider variety of acid-base reactions. Lewis' theory used electrons instead of proton transfer and specifically stated that an acid is a species that accepts an electron pair while a base donates an electron pair. Figure 1 Above: A Lewis Base (B) donates it electrons to a Lewis Acid (A) resulting in a coordinate covalently bonded compound, also known as an adduct. The reaction of a Lewis acid and a Lewis base will produce a coordinate covalent bond, as shown in Figure 1 above. A coordinate covalent bond is just a type of covalent bond in which one reactant gives it electron pair to another reactant. -

Abstract Reduction of Ketones to Alcohols And
ABSTRACT REDUCTION OF KETONES TO ALCOHOLS AND TERTIARY AMINES USING 1-HYDROSILATRANE Sami E. Varjosaari, Ph.D. Department of Chemistry and Biochemistry Northern Illinois University, 2018 Marc J. Adler, Co-Director Thomas M. Gilbert, Co-Director 1-Hydrosilatrane is an easily synthesized, stable, solid silane which can be made into an efficient reducing agent in the presence of a Lewis base, by taking advantage of the properties of hypervalent silicon. Due to the simplicity of use and handling, 1-hydrosilatrane has the potential to be an appealing alternative to other more widely used reducing agents. Aromatic and aliphatic ketones were readily reduced with 1-hydrosilatrane in the presence of potassium tert-butoxide. The discovery of diastereoselectivity in the reduction of menthone, a chiral ketone, led to enantioselective reductions of prochiral ketones using chiral Lewis base activators. Enantiomeric excesses of up to 86% were observed. It was also shown that 1-hydrosilatrane could act as a chemoselective reducing agent in the formation of tertiary amines via direct reductive aminations, in the absence of activator or solvent. Attempts were also made to take advantage of the steric properties of silicon protecting groups in meta-directing electrophilic aromatic substitution of phenols, leading to a one-pot synthesis of O-aryl carbamates. i NORTHERN ILLINOIS UNIVERSITY DEKALB, ILLINOIS MAY 2018 REDUCTION OF KETONES TO ALCOHOLS AND TERTIARY AMINES USING 1-HYDROSILATRANE BY SAMI ENSIO VARJOSAARI ©2018 SAMI ENSIO VARJOSAARI A DISSERTATION SUBMITTED TO THE GRADUATE SCHOOL IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE DOCTOR OF PHILOSOPHY DEPARTMENT OF CHEMISTRY AND BIOCHEMISTRY Doctoral Co-Directors: Marc J. -

Nature Vol 140-N3534.Indd
140 NATURE jl:LY 24, 1937 Obituary Notices Prof. H. E. Armstrong, F.R.S. College, South Kensington, where a novel type of ENRY EDWARD ARMSTRONG, whose death institution came into being, self-governed academic H on July 13 in his ninetieth year we regret to ally by its four professors-{)hemistry, engineering, announce, was born on May 6, 1848, in Lewisham, mathematics and physics-and independent of ex where he lived all his life. Of the influences during ternal examiners, which rapidly grew to be recognized his early youth which inclined him to chemistry, as a great professional school of engineering. But in little is known, but when he left Colfe's Grammar 1911, when the City and Guilds College by incorpora School at Lewisham his father, acting on the advice tion became the engineering section of the Imperial of an engineering friend, allowed him to become a College of Science and Technology, its chemistry and student at the Royal College of Chemistry, Oxford mathematics departments were closed down, although Street, in the summer term of 1865, actually Hof provision was made for chemistry diploma students mann's last term before his departure for Berlin. to finish their course under Armstrong's supervision Chemistry was the only subject taught, but the with its completion, chemistry ceased to be taught College was affiliated to the Royal School of Mines, in the City and Guilds College, and the last of the then housed in Jermyn Street. A free lance, he laboratories of the department was dismantled in attended such courses at the two institutions as he 1914. -
Frederick W. Westaway and Science Education: an Endless Quest
This is a repository copy of Frederick W. Westaway and science education: an endless quest. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/83231/ Version: Accepted Version Book Section: Jenkins, EW and Brock, WH (2014) Frederick W. Westaway and science education: an endless quest. In: Matthews, MR, (ed.) International Handbook of Research in History, Philosophy and Science Teaching. Springer Netherlands , 2359 - 2382. ISBN 978-94-007-7653-1 https://doi.org/10.1007/978-94-007-7654-8 Reuse Unless indicated otherwise, fulltext items are protected by copyright with all rights reserved. The copyright exception in section 29 of the Copyright, Designs and Patents Act 1988 allows the making of a single copy solely for the purpose of non-commercial research or private study within the limits of fair dealing. The publisher or other rights-holder may allow further reproduction and re-use of this version - refer to the White Rose Research Online record for this item. Where records identify the publisher as the copyright holder, users can verify any specific terms of use on the publisher’s website. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Frederick W. Westaway and Science Education: An Endless Quest WILLIAM H. BROCK and EDGAR W. JENKINS Department of History, University of Leicester, Leicester, UK. [email protected] School of Education, University of Leeds, Leeds, UK. -

Holmyard and the Historical Approach to Science Teaching.Pdf
This is a repository copy of E.J.Holmyard and the historical approach to science teaching. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/83229/ Version: Accepted Version Book Section: Jenkins, EW (2014) E.J.Holmyard and the historical approach to science teaching. In: International Handbook of Research in History, Philosophy and Science Teaching. Springer Netherlands , 2283 - 2408. ISBN 978-94-007-7653-1 https://doi.org/10.1007/978-94-007-7654-8 Reuse Unless indicated otherwise, fulltext items are protected by copyright with all rights reserved. The copyright exception in section 29 of the Copyright, Designs and Patents Act 1988 allows the making of a single copy solely for the purpose of non-commercial research or private study within the limits of fair dealing. The publisher or other rights-holder may allow further reproduction and re-use of this version - refer to the White Rose Research Online record for this item. Where records identify the publisher as the copyright holder, users can verify any specific terms of use on the publisher’s website. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ E. J. Holmyard (1891-1959) and the historical approach to science teaching © E.W.Jenkins, 2012 1 E.J.Holmyard (1891-1959) and the historical approach to science teaching Edgar W. Jenkins Centre for Studies in Science and Mathematics Education, University of Leeds, Leeds, UK.