Acid-Base Concepts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wastewater Technology Fact Sheet: Ammonia Stripping
United States Office of Water EPA 832-F-00-019 Environmental Protection Washington, D.C. September 2000 Agency Wastewater Technology Fact Sheet Ammonia Stripping DESCRIPTION Ammonia stripping is a simple desorption process used to lower the ammonia content of a wastewater stream. Some wastewaters contain large amounts of ammonia and/or nitrogen-containing compounds that may readily form ammonia. It is often easier and less expensive to remove nitrogen from wastewater in the form of ammonia than to convert it to nitrate-nitrogen before removing it (Culp et al., 1978). Ammonia (a weak base) reacts with water (a weak acid) to form ammonium hydroxide. In ammonia stripping, lime or caustic is added to the wastewater until the pH reaches 10.8 to 11.5 standard units which converts ammonium hydroxide ions to ammonia gas according to the following reaction(s): + - NH4 + OH 6 H2O + NH38 Source: Culp, et. al, 1978. Figure 1 illustrates two variations of ammonia FIGURE 1 TWO TYPES OF STRIPPING stripping towers, cross-flow and countercurrent. In TOWERS a cross-flow tower, the solvent gas (air) enters along the entire depth of fill and flows through the packing, as the alkaline wastewater flows it may be more economical to use alternate downward. A countercurrent tower draws air ammonia removal techniques, such as steam through openings at the bottom, as wastewater is stripping or biological methods. Air stripping may pumped to the top of a packed tower. Free also be used to remove many hydrophobic organic ammonia (NH3) is stripped from falling water molecules (Nutrient Control, 1983). droplets into the air stream, then discharged to the atmosphere. -
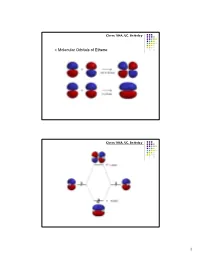
Π Molecular Orbitals of Ethene
Chem 104A, UC, Berkeley Molecular Orbitals of Ethene Chem 104A, UC, Berkeley 1 Chem 104A, UC, Berkeley Molecular Orbital Analysis of Ethene Dimerisation the reaction is said to be a "symmetry forbidden" – interestingly, this reaction is rare and very slow ! Chem 104A, UC, Berkeley Molecular Orbitals of 1,3-Butadiene 2 Chem 104A, UC, Berkeley Chem 104A, UC, Berkeley Molecular Orbital Analysis of Diels-Alder reaction 3 Chem 104A, UC, Berkeley Acid-Base Chemistry Reading : MT 6 Chem 104A, UC, Berkeley The Acid Base Theory of Brønsted and Lowry In 1923, within several months of each other, Johannes Nicolaus Brønsted (Denmark) and Thomas Martin Lowry (England) published essentially the same theory about how acids and bases behave. •An acid is a "proton donor." •A base is a "proton acceptor." 4 Chem 104A, UC, Berkeley + HCl + H2O <===> H3O + Cl¯ HCl - this is an acid, because it has a proton available to be transfered. H2O - this is a base, since it gets the proton that the acid lost. Now, here comes an interesting idea: + H3O - this is an acid, because it can give a proton. Cl¯ - this is a base, since it has the capacity to receive a proton. + Notice that each pair (HCl and Cl¯ as well as H2O and H3O differ by one proton (symbol = H+). These pairs are called conjugate pairs. Chem 104A, UC, Berkeley HCl + NaOH ---> H2O+ NaCl stronger stronger Weaker weaker conjugate conjugate acid base acid base + - NH4 +OH---> H2O+ NH3 stronger stronger weaker weaker conjugate conjugate acid base acid base 5 Chem 104A, UC, Berkeley H n A H 2O H n1 A H3O [H n1 A ][H3O ] pKa1 log [H n A] pKa 0 strong pKa 0 weak Chem 104A, UC, Berkeley Amphoteric Compound H n A H 2O H n1 A H3O NH 3 H 2O NH 4 OH 6 Chem 104A, UC, Berkeley Oxyacids AOp(OH)q A=Si, N, P, As, S, Se, Te, Cl, Br, I p=# of nonhydrogenated oxygen atom H + A ----O As atom A is rendered more positive, it becomes easier to break the O-H bond Because of enhanced bond polarization. -

Properties of Acids and Bases
GREEN CHEMISTRY LABORATORY MANUAL Lab 22 Properties of Acids and Bases TN Standard 4.2: The student will investigate the characteristics of acids and bases. Have you ever brushed your teeth and then drank a glass of orange juice? hat do you taste when you brush your teeth and drink orange juice afterwards. Yuck! It leaves a really bad taste in your mouth, but why? Orange juice and toothpaste by themselves taste good. But the terrible taste W results because an acid/base reaction is going on in your mouth. Orange juice is a weak acid and the toothpaste is a weak base. When they are placed together they neutralize each other and produce a product that is unpleasant to taste. How do you determine what is an acid and what is a base? In this lab we will discover how to distinguish between acids and bases. Introduction Two very important classes of compounds are acids and bases. But what exactly makes them different? There are differences in definition, physical differences, and reaction differences. According to the Arrhenius definition, acids ionize in water to + produce a hydronium ion (H3O ), and bases dissociate in water to produce hydroxide ion (OH -). Physical differences can be detected by the senses, including taste and touch. Acids have a sour or tart taste and can produce a stinging sensation to broken skin. For example, if you have ever tasted a lemon, it can often result in a sour face. Bases have a bitter taste and a slippery feel. Soap and many cleaning products are bases. -

Acids Lewis Acids and Bases Lewis Acids Lewis Acids: H+ Cu2+ Al3+ Lewis Bases
E5 Lewis Acids and Bases (Session 1) Acids November 5 - 11 Session one Bronsted: Acids are proton donors. • Pre-lab (p.151) due • Problem • 1st hour discussion of E4 • Compounds containing cations other than • Lab (Parts 1and 2A) H+ are acids! Session two DEMO • Lab: Parts 2B, 3 and 4 Problem: Some acids do not contain protons Lewis Acids and Bases Example: Al3+ (aq) = ≈ pH 3! Defines acid/base without using the word proton: + H H H • Cl-H + • O Cl- • • •• O H H Acid Base Base Acid . A BASE DONATES unbonded ELECTRON PAIR/S. An ACID ACCEPTS ELECTRON PAIR/S . Deodorants and acid loving plant foods contain aluminum salts Lewis Acids Lewis Bases . Electron rich species; electron pair donors. Electron deficient species ; potential electron pair acceptors. Lewis acids: H+ Cu2+ Al3+ “I’m deficient!” Ammonia hydroxide ion water__ (ammine) (hydroxo) (aquo) Acid 1 Lewis Acid-Base Reactions Lewis Acid-Base Reactions Example Metal ion + BONDED H H H + • to water H + • O O • • •• molecules H H Metal ion Acid + Base Complex ion surrounded by water . The acid reacts with the base by bonding to one molecules or more available electron pairs on the base. The acid-base bond is coordinate covalent. The product is a complex or complex ion Lewis Acid-Base Reaction Products Lewis Acid-Base Reaction Products Net Reaction Examples Net Reaction Examples 2+ 2+ + + Pb + 4 H2O [Pb(H2O)4] H + H2O [H(H2O)] Lewis acid Lewis base Tetra aquo lead ion Lewis acid Lewis base Hydronium ion 2+ 2+ 2+ 2+ Ni + 6 H2O [Ni(H2O)6] Cu + 4 H2O [Cu(H2O)4] Lewis acid Lewis base Hexa aquo nickel ion Lewis acid Lewis base Tetra aquo copper(II)ion DEMO DEMO Metal Aquo Complex Ions Part 1. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -

Lesson 21: Acids & Bases
Lesson 21: Acids & Bases - Far From Basic Lesson Objectives: • Students will identify acids and bases by the Lewis, Bronsted-Lowry, and Arrhenius models. • Students will calculate the pH of given solutions. Getting Curious You’ve probably heard of pH before. Many personal hygiene products make claims about pH that are sometimes based on true science, but frequently are not. pH is the measurement of [H+] ion concentration in any solution. Generally, it can tell us about the acidity or alkaline (basicness) of a solution. Click on the CK-12 PLIX Interactive below for an introduction to acids, bases, and pH, and then answer the questions below. Directions: Log into CK-12 as follows: Username - your Whitmore School Username Password - whitmore2018 Questions: Copy and paste questions 1-3 in the Submit Box at the bottom of this page, and answer the questions before going any further in the lesson: 1. After dragging the small white circles (in this simulation, the indicator papers) onto each substance, what do you observe about the pH of each substance? 2. If you were to taste each substance (highly inadvisable in the chemistry laboratory!), how would you imagine they would taste? 3. Of the three substances, which would you characterize as acid? Which as basic? Which as neutral? Use the observed pH levels to support your hypotheses. Chemistry Time Properties of Acids and Bases Acids and bases are versatile and useful materials in many chemical reactions. Some properties that are common to aqueous solutions of acids and bases are listed in the table below. Acids Bases conduct electricity in solution conduct electricity in solution turn blue litmus paper red turn red litmus paper blue have a sour taste have a slippery feeling react with acids to create a neutral react with bases to create a neutral solution solution react with active metals to produce hydrogen gas Note: Litmus paper is a type of treated paper that changes color based on the acidity of the solution it comes in contact with. -

Drugs and Acid Dissociation Constants Ionisation of Drug Molecules Most Drugs Ionise in Aqueous Solution.1 They Are Weak Acids Or Weak Bases
Drugs and acid dissociation constants Ionisation of drug molecules Most drugs ionise in aqueous solution.1 They are weak acids or weak bases. Those that are weak acids ionise in water to give acidic solutions while those that are weak bases ionise to give basic solutions. Drug molecules that are weak acids Drug molecules that are weak bases where, HA = acid (the drug molecule) where, B = base (the drug molecule) H2O = base H2O = acid A− = conjugate base (the drug anion) OH− = conjugate base (the drug anion) + + H3O = conjugate acid BH = conjugate acid Acid dissociation constant, Ka For a drug molecule that is a weak acid The equilibrium constant for this ionisation is given by the equation + − where [H3O ], [A ], [HA] and [H2O] are the concentrations at equilibrium. In a dilute solution the concentration of water is to all intents and purposes constant. So the equation is simplified to: where Ka is the acid dissociation constant for the weak acid + + Also, H3O is often written simply as H and the equation for Ka is usually written as: Values for Ka are extremely small and, therefore, pKa values are given (similar to the reason pH is used rather than [H+]. The relationship between pKa and pH is given by the Henderson–Hasselbalch equation: or This relationship is important when determining pKa values from pH measurements. Base dissociation constant, Kb For a drug molecule that is a weak base: 1 Ionisation of drug molecules. 1 Following the same logic as for deriving Ka, base dissociation constant, Kb, is given by: and Ionisation of water Water ionises very slightly. -

Acids and Bases
self study Most of this is probably background from a prior chemistry course; some near the end is GG325 review Aqueous Inorganic Geochemistry of Natural Waters Three important topics: 1. Acids, bases and the aqueous CO 2 system 2. Behavior of ions in aqueous solution 3. Quantifying aqueous solubility and total dissolved solids (TDS) Pease read Manahan chapter 3 and review chapter 28 (6 th Ed) for next week GG425 Wk2, S2016 Acids and Bases GG425 Wk2, S2016 1 1. Two Types of Acids and Bases: a. Brönstead acids and bases contain H + and OH - HCl ↔ H + + + Cl- (acid) NaOH ↔ Na + + OH - (base). + - water is acid and base simultaneously, H 2O ↔ H + OH . pure water and acid neutral aqueous solutions have equal amounts of H + and OH -. b. Lewis acids and bases Brönstead acids can be thought of as electron deficient ions and bases as electron excessive ions , which provides a different perspective on acidity/basisity that we can extend to other (non protic and non hydroxyl) compounds. We call this the Lewis acid/base concept. GG425 Wk2, S2016 Brönstead acidity: Kw is the equilibrium constant for the dissociation of water. + - H2O ↔ H + OH + - -14 Kw = [H ][OH ] = 10 at 25 EC Kw has a slight temperature dependence: Temp ( EC) Kw 0 10 -14.94 less dissociated 25 10 -14 60 10 -13.02 more dissociated GG425 Wk2, S2016 2 Brönstead acidity: + - H2O ↔ H + OH + - Kw = [H ][OH ] An acid neutral solution always has [H +] = [OH -]. Setting [H +] = x, yields x 2= 10 -14 x = 10 -7 = moles of H + in this solution. -

Notes Acids and Bases
Notes Acids and Bases Arrhenius Acid Base Theory: In 1884 Svante Arrhenius proposed the first theoretical model for acids and bases. Prior to that time, these chemically opposite substances were described in properties such as their taste; their effects on metals, carbonates, and dyes (called indicators); their feel to the touch, and their ability to react with each other. According to the Arrhenius theory , pure water dissociates to some extent to produce hydrogen ions, H + and hydroxide ions, OH -. When this occurs, equal amounts of H + and OH - ions are produced: + - H2O(l) H (aq) + OH (aq) An acid, according to Arrhenius, is any substance that liberates H + ions when placed in water. When the H + concentration is elevated the OH - concentration decreases, this solution is said to be acidic. Similarly, a base is defined as any substance that liberates OH - ions when placed in water. The resulting solution has a higher concentration of OH - ions than H + ions and is said to be basic, or alkaline. + - ACID: HCl (aq) H (aq) + Cl (aq) + - BASE: NaOH (aq) Na (aq) + OH (aq) Brønsted - Lowry Acid Base Theory: A more general and realistic theory was proposed by two chemists working independent of the other, Johannes Nicolaus Brønsted of Denmark and Thomas Martin Lowry of England. Both chemists are given credit; the theory is named the Brønsted-Lowry theory . Acids are defined as substances that donate H + ions, protons and therefore are called proton donators, while bases are substances that accept protons and are defined as proton acceptors. On this basis, the autoionization of water is given as follows: + - H2O(l) + H 2O(l) H3O (aq) + OH (aq) In this reaction, one water molecule donates a proton, H +, and behaves as an acid, while the other water molecule accepts the proton and behaves as a base. -

Chem331 Lect 2 Water Ph Acid Base Buffer
Chapter 2 – Water and pH Water - one of the most important molecules in life. •70% of the bodies mass is water •2/3 of total body water is intracellular (55-66% body weight of men and 10% less for women) •The rest is interstitial fluid of which 25% is in the blood plasma. pH - The body tightly controls both the volume and pH of water. •The bicarbonate system is crucial for blood maintenance •changes of pH greater than 0.1 are dangerous and can lead to coma -diabetics Properties of water • Polarity • Hydrogen bonding potential Specific heat, heat of vaporization • It is the unique combination of properties of water • Nucleophilic that make it the perfect solvent for biological • Ionization systems. We will discuss each of these properties in • Water is an ideal more detail. biological solvent Water is close to a tetrahedral shape with the unshared electrons on the two sp3-hybridized orbital are in two corners and the hydrogen in others o Compared to a tetrahedron, CH4 (109 ) or NH3 the bond angle is smaller (109.5o and 107o vs.104.5o) Water has hydrogen bonding potential •H-bonds are non-covalent, weak interactions •H2O is both a Hydrogen donor and acceptor •One H2O can form up to four H-bonds What Are the Properties of Water? A comparison of ice and water, in terms of H-bonds and Motion • Ice: 4 H bonds per water molecule • Water: 2.3 H bonds per water molecule • Ice: H-bond lifetime - about 10 microsec • Water: H-bond lifetime - about 10 psec • (10 psec = 0.00000000001 sec) The Solvent Properties of Water Derive from Its Polar Nature •Water has a high dielectric constant •Ions are always hydrated in water and carry around a "hydration shell" •Water forms H bonds with polar solutes The Solvent Properties of Water Derive from Its Polar Nature What makes this molecule important? solvent ability - easily disrupts ionic compounds - dielectric constant (D) is high (measure of the ability to keep ions apart) – Large electronegativity creates a strong ionic type bond (dipole). -

Proton Affinity Changes Driving Unidirectional Proton Transport In
doi:10.1016/S0022-2836(03)00903-3 J. Mol. Biol. (2003) 332, 1183–1193 Proton Affinity Changes Driving Unidirectional Proton Transport in the Bacteriorhodopsin Photocycle Alexey Onufriev1, Alexander Smondyrev2 and Donald Bashford1* 1Department of Molecular Bacteriorhodopsin is the smallest autonomous light-driven proton pump. Biology, The Scripps Research Proposals as to how it achieves the directionality of its trans-membrane Institute, 10550 North Torrey proton transport fall into two categories: accessibility-switch models in Pines Road, La Jolla, CA 92037 which proton transfer pathways in different parts of the molecule are USA opened and closed during the photocycle, and affinity-switch models, which focus on changes in proton affinity of groups along the transport 2Schro¨dinger Inc., 120 West chain during the photocycle. Using newly available structural data, and Forty-Fifth Street, 32nd Floor adapting current methods of protein protonation-state prediction to the Tower 45, New York, NY non-equilibrium case, we have calculated the relative free energies of pro- 10036-4041, USA tonation microstates of groups on the transport chain during key confor- mational states of the photocycle. Proton flow is modeled using accessibility limitations that do not change during the photocycle. The results show that changes in affinity (microstate energy) calculable from the structural models are sufficient to drive unidirectional proton trans- port without invoking an accessibility switch. Modeling studies for the N state relative to late M suggest that small structural re-arrangements in the cytoplasmic side may be enough to produce the crucial affinity change of Asp96 during N that allows it to participate in the reprotonation of the Schiff base from the cytoplasmic side. -

Proton Affinity of SO3
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Proton Affinity of SO3 Cynthia Ann Pommerening, Steven M. Bachrach, and Lee S. Sunderlin Department of Chemistry, Northern Illinois University, DeKalb, Illinois, USA ϩ Collision-induced dissociation (CID) of the radical cation H2SO4 gives the product pairs ϩ ϩ ϩ ϩ H2O SO3 and HO HSO3 with a 1:3 ratio that is essentially independent of collision energy. Statistical analysis of the two channels indicates that the proton affinity of HO is 3 Ϯ ϭ Ϯ 4 kJ/mol lower than that of SO3. This can be used to derive PA(SO3) 591 4 kJ/mol at 0 K and 596 Ϯ 4 kJ/mol at 298 K. Previously, Munson and Smith bracketed the proton affinity as PA(HBr) ϭ 584 kJ/mol Ͻ PA(SO ) Ͻ PA(CO) ϭ 594 kJ/mol. The threshold of 152 Ϯ 16 ϩ 3 kJ/mol for formation of H O ϩ SO indicates that the barrier to CID is small or nonexistent, 2 3 ϩ in contrast to the substantial barriers to decomposition for H3SO4 and H2SO4. (JAmSoc Mass Spectrom 1999, 10, 856–861) © 1999 American Society for Mass Spectrometry he development of extensive scales of proton this work provide an independent measurement of the ⌬ affinity (PA), gas basicity (GB), and acidity ( Ha) PA of SO3. values has provided a framework for the quanti- The gas-phase addition of H2OtoSO3 to form T ϭϪ⌬ tative understanding of ion properties. (PA H for sulfuric acid has a substantial barrier, as indicated by addition of a proton, GB ϭϪ⌬G for addition of a reaction rate measurements [4–6] and computational ⌬ ϭ⌬ proton, and Ha H for deprotonation.) The history results [7, 8].